Method for checking oral traditional Chinese medicine formulation and applications thereof
A detection method and a technology for oral preparations, applied in the field of analytical chemistry, to achieve the effects of good repeatability, good logarithmic linearity and good intermediate precision
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 :Identification of Duerwei Capsules of the Invention
[0046] This example is to identify Duyiwei and Panax notoginseng in the Duerwei capsule of the present invention. In the following, combined with the characteristics of this product, a thin-layer chromatography identification study was carried out on Duerwei capsules (sample batch numbers: 080301, 080302, 080303). The Duerwei control medicinal material was used as a control to identify the product, and different development systems were used to investigate its thin layer. Expand behavior.
[0047] 1. Unique identification method
[0048] Preparation of test solution: Take 1.0g of the contents of this product, grind it, add 15ml of ethanol, heat to reflux for 15 minutes, take it out, filter, and use the filtrate as the test solution.
[0049] The preparation of negative sample solution (lack of Duyiwei): According to the manufacturing method of Duerwei capsules, prepare a capsule sample that does not contain ...
Embodiment 2
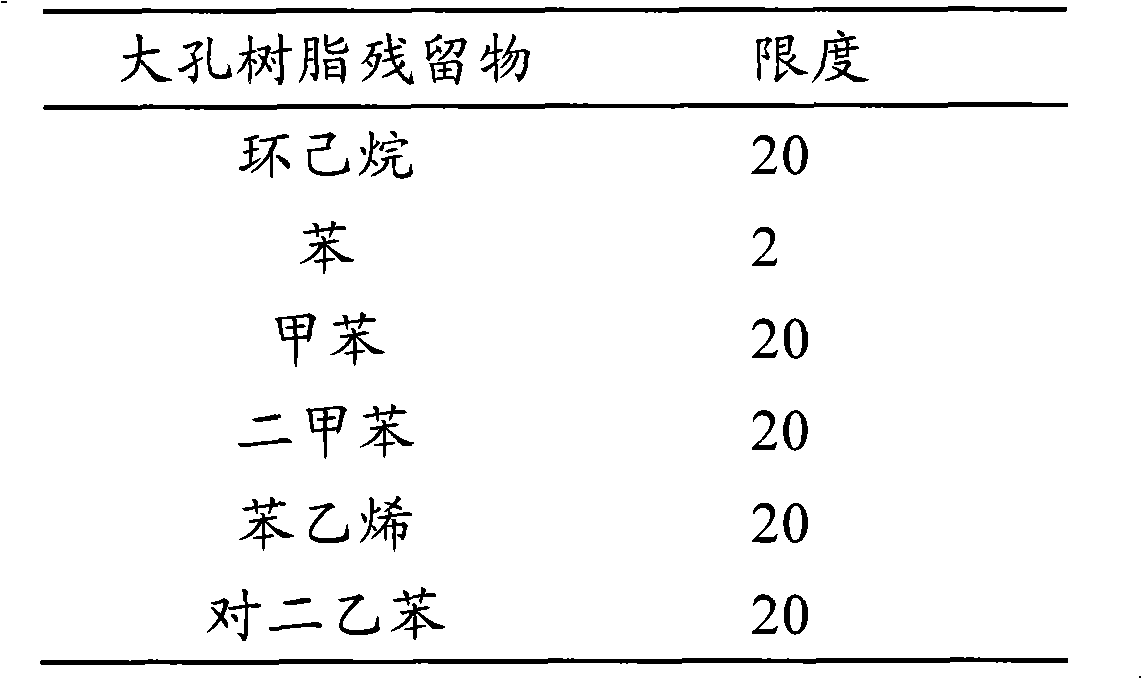
[0056] Example 2 :Resin residues of the Duerwei capsule of the present invention
[0057] According to the "Technical Requirements for Separation and Purification of Chinese Medicine Extracts by Macroporous Adsorption Resin (Interim)" issued by the National Medical Products Administration with Yaoguanzhu [2000] No. 56 on February 17, 2000, it is necessary to deal with large styrene framework Porous resin residue inspection items are benzene, toluene, xylene, styrene, alkanes, diethylbenzene (divinyl) and other organic residues that may be introduced by resin. The limit cannot be higher than the national standard. Or international standards. The residues that may be introduced by the styrene skeleton type macroporous resin used in the process of Duerwei capsules and their limits are shown in Table 1.
[0058] Table 1 Introduced resin residue limit results
[0059]
[0060] The residues introduced by the styrene skeleton type macroporous resin need to be controlled to a limit du...
Embodiment 3
[0112] Example 3 :Determination of the main ingredient content of the Duerwei capsule of the present invention
[0113] This example is the determination of the content of the main ingredients of the Duerwei capsule of the present invention. Duerwei Capsules are processed from Duyiwei and Panax notoginseng. With reference to the pharmacopoeia and related literature, high performance liquid chromatography (HPLC method) is used to determine the luteolin and the saponins in the Panax notoginseng. The content of the ingredients is used as a control method.
[0114] 1. Determination of luteolin content
[0115] (1) Instruments and reagents
[0116] Instrument: Agilent 1100 High Performance Liquid Chromatograph and supporting workstation
[0117] Sartorius CP225D electronic balance
[0118] Luteolin reference substance batch number: 111520-200504, for content determination, purchased from the China Inspection Institute.
[0119] Reagents: chromatographic methanol is chromatographically...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| separation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com