Fusion heparinase and coding gene and preparation method thereof
A technology of heparinase and gene, applied in the fields of enzyme engineering and biocatalysis, can solve the problems of lack of integrated methods for enzyme production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
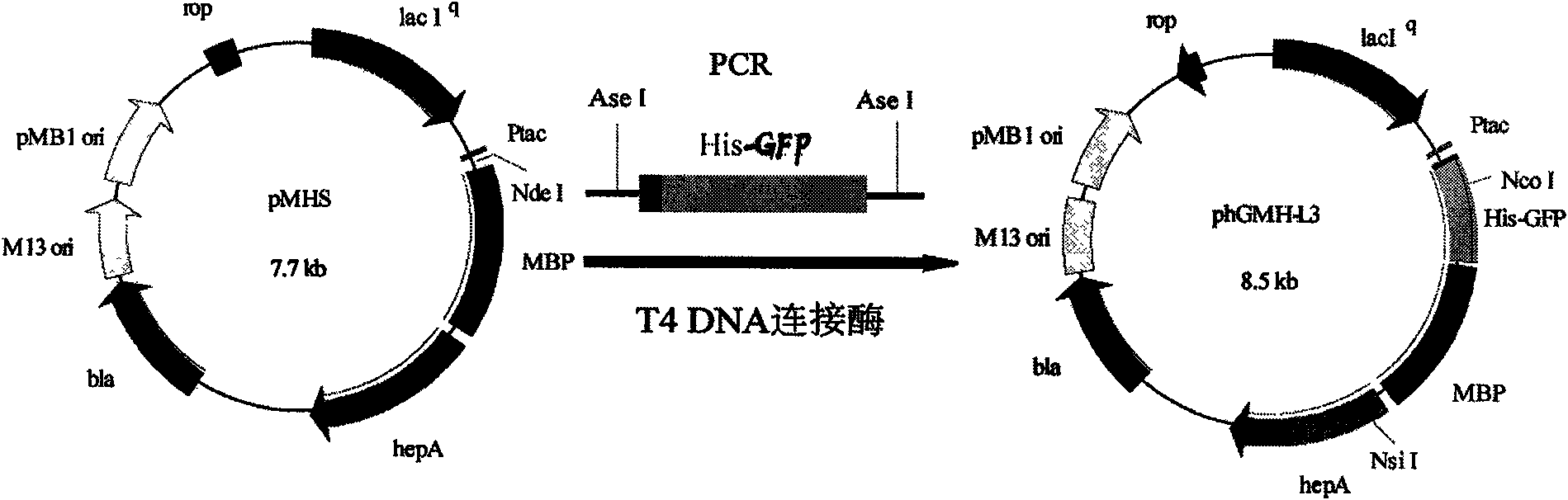
[0040] Example 1. Expression of triple fusion protein of green fluorescent protein, maltose binding protein and heparanase I
[0041] 1. Construction of the expression vector of the triple fusion protein
[0042] 1. Construction of the expression vector pMAL-c2x-HepA containing the gene encoding the double fusion protein of maltose binding protein and heparanase I
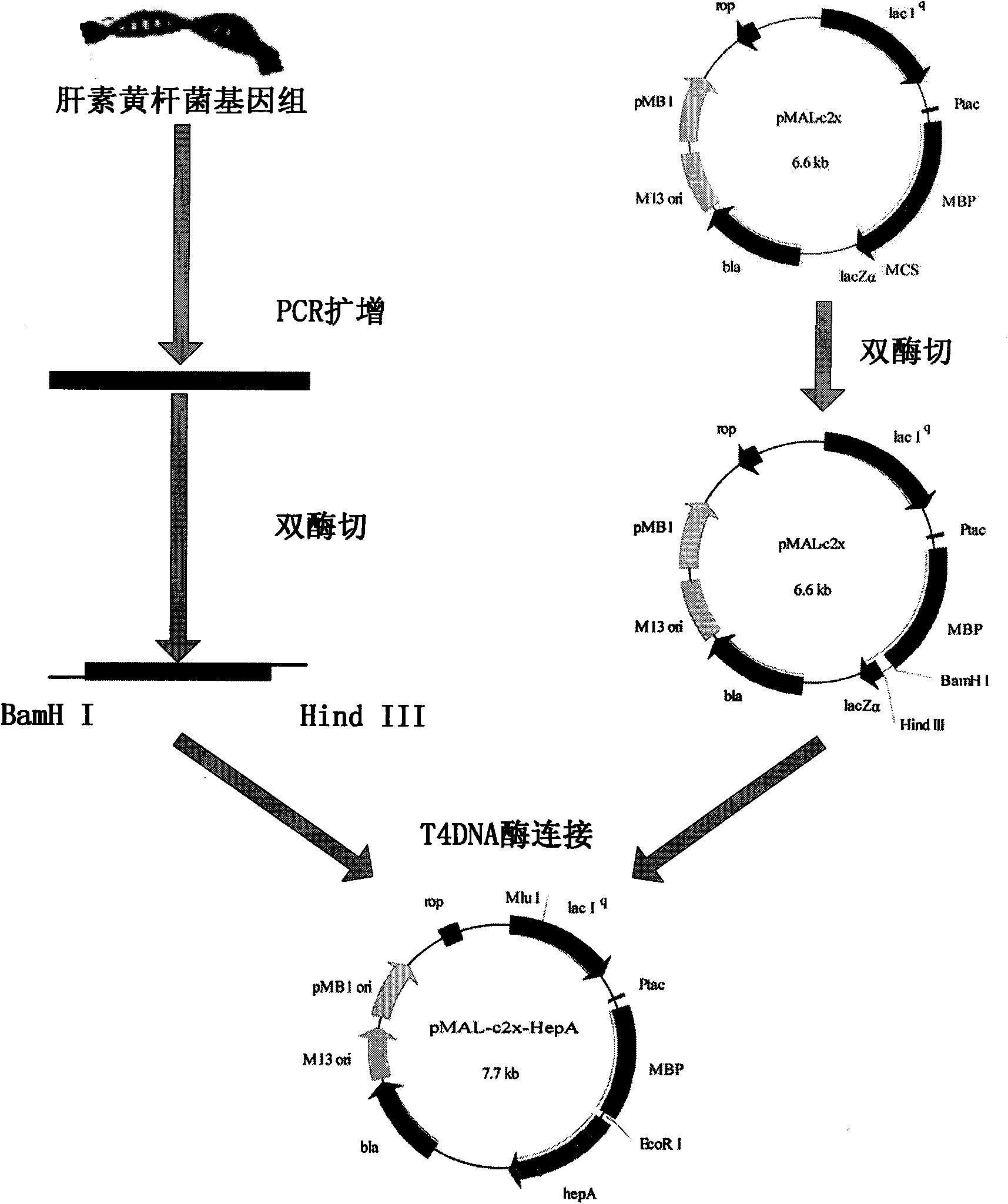
[0043] The construction process of the expression vector pMAL-c2x-HepA is as follows: figure 1 As shown, the specific process is as follows: amplify the heparanase I gene from the genomic DNA of Flavabacterium heparinum (Flavabacterium heparinum) (purchased from IAM), and the primers used are respectively:
[0044] Upstream primer: 5′-GCCT GGATCC CAGCAAAAAAAAATCCGGTAAC-3' (the underlined base is the enzyme recognition site of BamHI),
[0045] Downstream primer: 5′-CTTA AAGCTT TTACTATCTGGCAGTTTCGCTGTAC-3' (bases underlined are HindIII enzyme recognition sites), after amplification, BamHI and HindIII enzyme rec...
Embodiment 2 3
[0064] Example 2. Fluorescence quantitative tracking of triple fusion protein heparanase activity
[0065] According to the results of Example 1, we selected Escherichia coli TOP10 / phGMH-L3 to conduct a fluorescence quantitative tracking experiment of the heparanase activity of the triple fusion protein. M9YE medium containing 100 μg / ml ampicillin (17.1 g / L Na 2 HPO 4 12H 2 O, 3.0g / L KH 2 PO 4 , 0.5g / L NaCl; 1.0g / L NH 4 Cl, 12.5g / L yeast extract, 12g / L glucose) to cultivate TOP10 / phGMH-L3 at 37°C for about 3.5 hours (to OD 600 0.735), add IPTG with a final concentration of 0.3mM, and change the culture temperature to 15°C for induction culture. After that, every 2 hours, take 2ml of bacterial solution to measure the cell concentration (OD 600 ), the enzyme activity and fluorescence intensity of heparanase I (fluorescence spectrophotometer HITACHI F-2500), until the induction culture for 30 hours.
[0066] The enzyme activity of heparanase I was determined according to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Extinction coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com