Application of rLZ-8 in treating thrombopenia and preparation thereof
A technique for thrombocytopenia and rlz-8, applied to the application of rLZ-8 in the treatment of thrombocytopenia and the field of preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
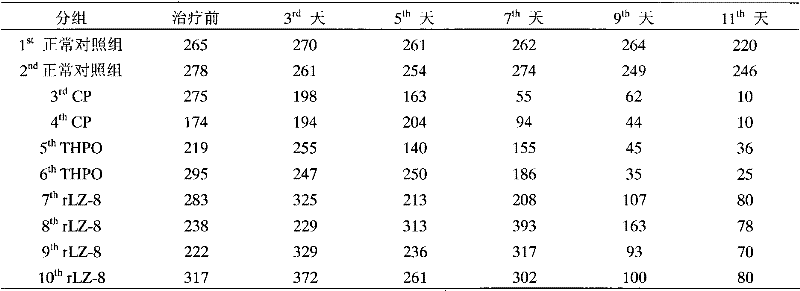
[0014] Example 1. The pharmacodynamics test of rLZ-8 preventing canine thrombocytopenia caused by cyclophosphamide
[0015] 1. Drug preparation:
[0016] Test drug: Recombinant Ganoderma lucidum immunomodulatory protein (rLZ-8) was prepared in 3.85 μg·kg sterile saline -1 , 1.925 μg kg -1 Dosage group, 1ml / piece.
[0017] Positive control drug: Thrombopoietin (THPO), produced by Shenyang Sansheng Pharmaceutical Co., Ltd., 20 μg kg -1 / d, 1ml / piece.
[0018] Chemotherapy drug: Cyclophosphamide (Cy), produced by Jiangsu Hengrui Pharmaceutical Co., Ltd., production batch number 08112121; 200mg / bottle. Accurately weigh 7mg·kg with an electronic scale -1 / Only.
[0019] Platelet diluent: urea: 1.3g, sodium citrate: 0.5g, formaldehyde: 0.1ml, add distilled water to 100ml and mix, filter for later use.
[0020] 2. Experimental method:
[0021] The experimental animals were divided into 5 groups, namely normal control group, model group (CP), positive drug control group and ex...
Embodiment 2
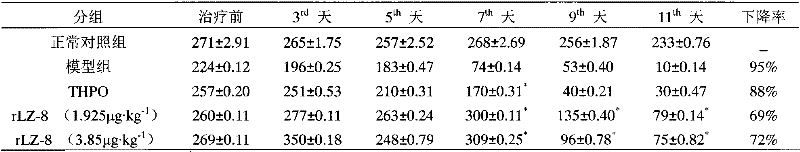
[0029] Example 2. The pharmacodynamics test of rLZ-8 in the treatment of canine thrombocytopenia caused by cyclophosphamide
[0030] 1. Drug preparation:
[0031] Test drug: Recombinant Ganoderma lucidum immunomodulatory protein (rLZ-8) was prepared in 3.85 μg·kg sterile saline -1 , 1.925 μg kg -1 Dosage group, 1ml / piece.
[0032] Positive control drug: Thrombopoietin (THPO), produced by Shenyang Sansheng Pharmaceutical Co., Ltd., 20 μg kg -1 / d, 1ml / piece.
[0033] Chemotherapy drug: Cyclophosphamide (Cy), produced by Jiangsu Hengrui Pharmaceutical Co., Ltd., production batch number 08112121; 200mg / bottle. Accurately weigh 7mg·kg with an electronic scale -1 / Only.
[0034] Platelet diluent: urea: 1.3g, sodium citrate: 0.5g, formaldehyde: 0.1ml, add distilled water to 100ml and mix, filter for later use.
[0035] 2. Experimental method:
[0036] The experimental animals were divided into 5 groups, namely normal control group, model group (CP), positive drug control gro...
Embodiment 3
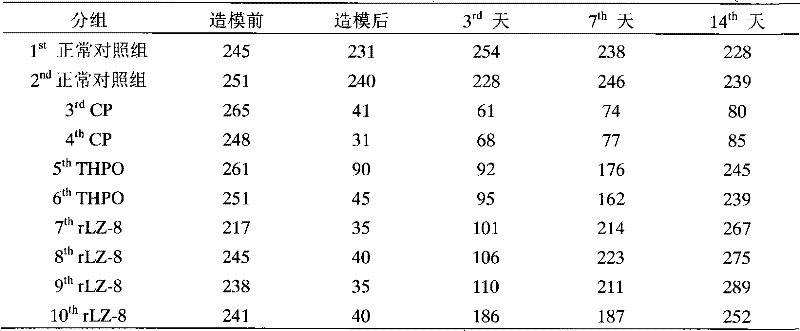
[0044] Example 3. The pharmacodynamics test of rLZ-8 preventing the mouse thrombocytopenia caused by cyclophosphamide
[0045] 1. Drug preparation:
[0046] Test drug: Recombinant Ganoderma lucidum immunomodulatory protein (rLZ-8) was prepared with sterile water for injection to 19.25 μg kg -1 , 9.625 μg kg -1 Dosage group, 0.2ml / piece.
[0047] Positive control drug: Thrombopoietin (THPO), produced by Shenyang Sansheng Pharmaceutical Co., Ltd., 770 μg kg -1 / d, 0.2ml / piece.
[0048] Chemotherapy drug: Cyclophosphamide (Cy), produced by Jiangsu Hengrui Pharmaceutical Co., Ltd., production batch number 08112121; 200mg / bottle. Prepare 100mg·kg with sterile water for injection -1 , 0.2ml / piece.
[0049] Platelet diluent: urea: 1.3g, sodium citrate: 0.5g, formaldehyde: 0.1ml, add distilled water to 100ml and mix, filter for later use.
[0050] 2. Experimental method:
[0051] Experimental animals were divided into 5 groups, 10 mice in each group, half male and half male. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com