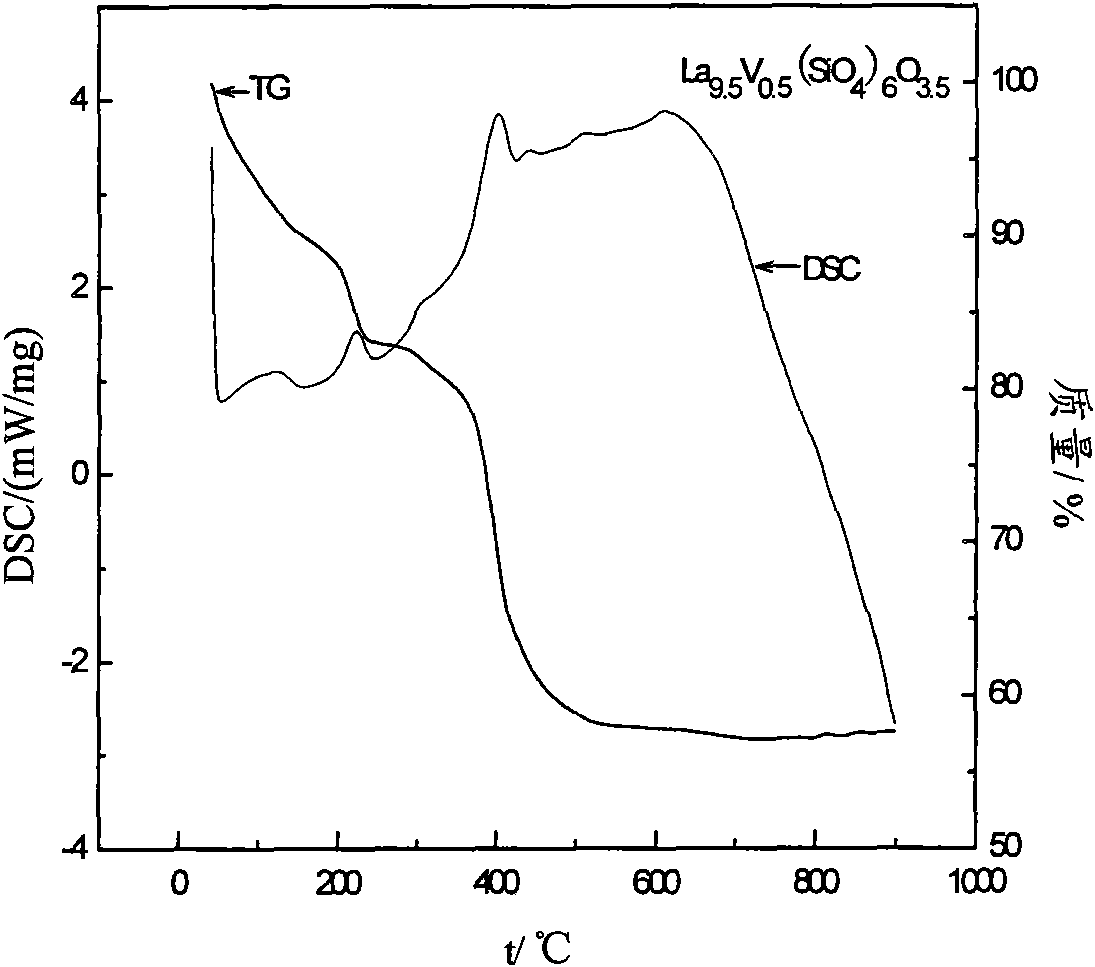
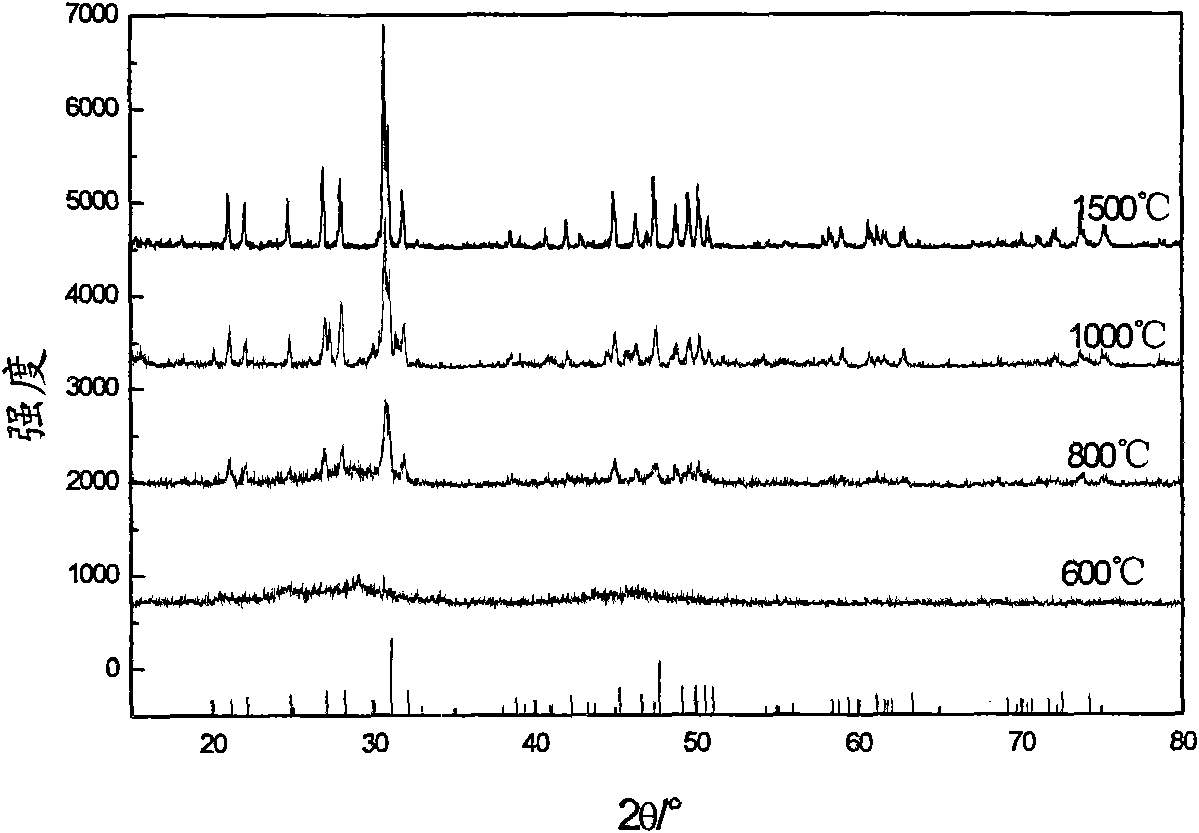
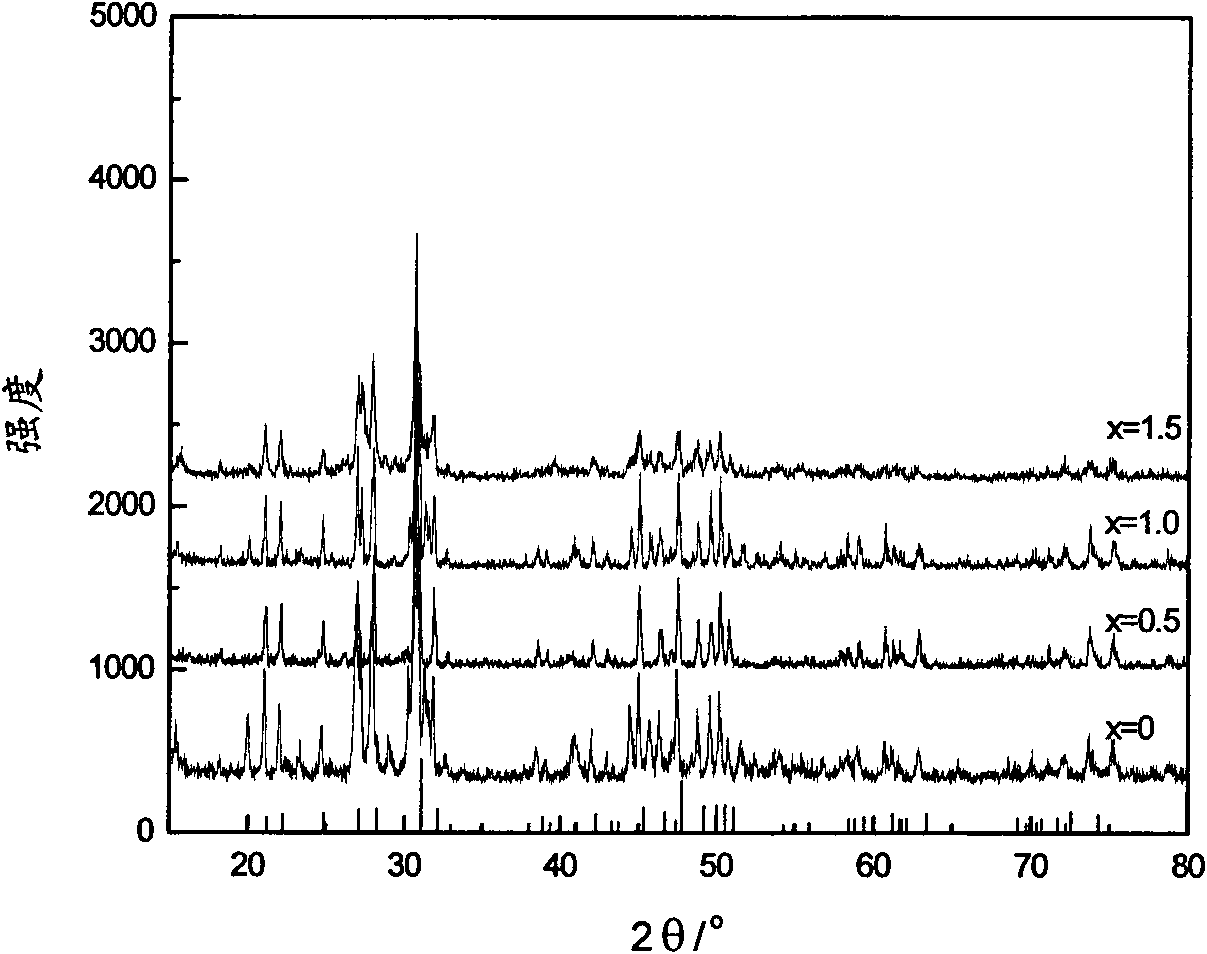
Vanadium-doped in lanthanum site apatite-type lanthanum silicate solid electrolyte material and preparation method thereof
A technology of solid electrolyte and apatite, which is applied in the direction of solid electrolyte fuel cells and fuel cell components, can solve the problems of complex preparation process, low polarization loss, and restrictions on large-scale application, and achieve high conductivity, The effect of extending the service life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Preparation of 0.002 mol La by the sol-gel method 9.5 V 0.5 (SiO 4 ) 6 O 3.5 Powder: Weigh La (NO 3 ) 3 ·6H 2 Put 8.2274g of O powder into a 100ml tall beaker, add 5.36ml of ethanol and 5.36ml of glacial acetic acid, stir until the powder is completely dissolved to obtain a colorless and transparent solution, then slowly add (about 30 minutes) 2.68ml of TEOS under stirring conditions , the obtained colorless and transparent solution was continuously stirred for 1h until the reaction was complete; at the same time, 0.1170g NH was dissolved in 2ml of dilute ammonia water with a concentration of 9.5% by weight. 4 VO 3 An orange solution was obtained. The orange solution was slowly added to the colorless and transparent solution under rapid stirring. The obtained orange solution was continuously stirred for 1 h. The orange solution was slowly stirred and evaporated at 80 °C until it was in a gel state. After drying at 130 °C, about 10 mg was weighed and heated. Gra...
Embodiment 2
[0034] Preparation of 0.002mol La by sol-gel method 9.0 V 1.0 (SiO 4 ) 6 o 4.0 Powder: weigh La(NO 3 ) 3 ·6H 2 Put 7.7944g of O powder into a 100ml tall beaker, add 5.36ml of ethanol and 5.36ml of glacial acetic acid, stir until the powder is completely dissolved to obtain a colorless transparent solution, then slowly add (about 30 minutes) 2.68ml of TEOS under stirring conditions , the obtained colorless and transparent solution was continuously stirred for 1h until the reaction was complete; at the same time, 0.2340gNH 4 VO 3 An orange solution was obtained. Slowly add the orange solution (20 minutes) into the colorless and transparent solution under rapid stirring, and the obtained orange solution continues to stir for 1 hour, slowly stir and evaporate the orange solution at 60°C until it is in a gel state, and then dry at 150°C and cool at 500°C Pre-baked for 5 hours, the fully ground powder was pressed into a membrane under a pressure of 10Mpa, and sintered at 10...
Embodiment 3
[0036] Preparation of 0.002mol La by sol-gel method 8.5 V 1.5 (SiO 4 ) 6 o 4.5 Powder: weigh La(NO 3 ) 3 ·6H 2 Put 7.3613g of O powder into a 100ml tall beaker, add 5.36ml of ethanol and 5.36ml of glacial acetic acid, stir until the powder is completely dissolved to obtain a colorless transparent solution, then slowly add (about 30 minutes) 2.68ml of TEOS under stirring conditions , the obtained colorless transparent solution was continuously stirred for 1h until the reaction was complete; at the same time, 0.3510gNH was dissolved in 10% dilute ammonia water with a concentration of 3ml 4 VO 3 An orange solution was obtained. Slowly add the orange solution (about 20 minutes) into the colorless and transparent solution under rapid stirring (600n / min), and the obtained orange solution continues to stir for 1h, and slowly stirs (30n / min) at 60°C to evaporate the orange solution until it solidifies. In the gel state, after drying at 150°C and pre-calcining at 400°C for 5 h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com