Liposome preparation device and method for preparing liposome by using same
A system and lipid technology, which is applied in the direction of liposome delivery, making drugs into special physical or taking forms, etc., can solve the difficulty of drug dispersion speed reaching the ideal level, the influence of liposome particle size, lipid Problems such as uneven particle size of plastids, to achieve the effect of excellent preparation reproducibility, narrow particle size distribution, and short production cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
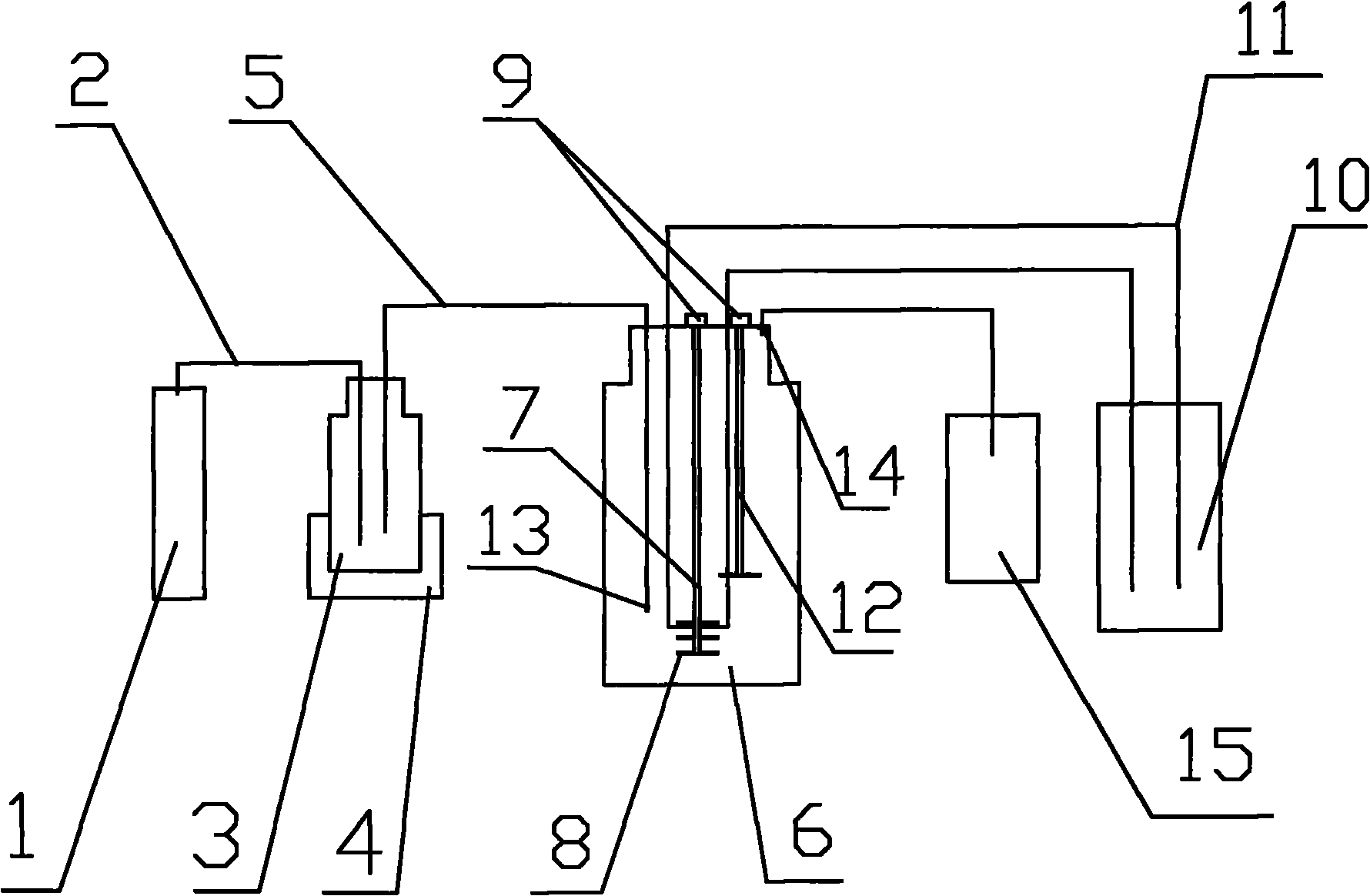
[0029]With the concentration of ethanol as solvent, lecithin and 100mg / ml cholesterol are mixed with a molar ratio of 1:1, and 500ml is dissolved in 1000ml of ethanol at 46°C to form an organic phase solution, and the organic phase solution Inject into the emulsification bottle 3; 2.5g of the polypeptide drug hepatitis B core antigen HBcAg18-27 is dissolved in 500ml of 50% ethanol at 46°C to form a drug solution with a concentration of 5mg / ml, and the organic The phase solution and the drug solution are mixed in the emulsification bottle 3. While mixing, nitrogen gas with a pressure of 1mpa is passed into the emulsification bottle, and the emulsion is kept at a constant temperature of 46°C for 20mins through the thermostat 4; after 20mins, the nitrogen gas is increased. Pressure, according to the speed of 1ml / s, the emulsification is hydraulically injected into the reactor 6, and 10L of sterilized pharmaceutical water with a temperature of 46°C is added to the reactor 6 in adva...
Embodiment 2
[0032] Mix lecithin with a concentration of 100mg / ml and 100mg / ml cholesterol with ethanol as a solvent in a molar ratio of 1:1, take 500ml and dissolve it in 1000ml of ethanol at 40°C to form an organic phase solution, and dissolve the organic phase solution Inject into emulsifying bottle 3; vitamin B 12 5g is dissolved in 500ml of 50% ethanol at 25°C to form a drug solution with a concentration of 5mg / ml, then the organic phase solution and the drug solution are mixed in the emulsification bottle 3 according to the volume ratio of 2:1, and when mixing, add Inject nitrogen gas with a pressure of 1mpa into the emulsification bottle, and maintain the temperature of the emulsion at 46°C for 20mins through the thermostat 4; after 20mins, increase the nitrogen pressure, and pump the emulsion into the reactor 6 at a speed of 1ml / s. 10L of sterilized pharmaceutical water with a temperature of 46°C was added to the reactor 6 in advance. During the process of injecting the emulsion in...
Embodiment 3
[0035] Mix lecithin with a concentration of 100mg / ml and 100mg / ml cholesterol with ethanol as a solvent in a molar ratio of 1:1, take 500ml and dissolve it in 1000ml of ethanol at 40°C to form an organic phase solution, and dissolve the organic phase solution Inject into the emulsification bottle 3; 2g of insulin is dissolved in 500ml of 50% ethanol at 25°C to form a drug solution with a concentration of 5mg / ml, and then the organic phase solution and the drug solution are mixed in the emulsification bottle according to the volume ratio of 2:1. 3. Mix in 3. While mixing, feed nitrogen gas with a pressure of 1mpa into the emulsification bottle, and keep the emulsified liquid at a constant temperature of 46°C for 20mins through a thermostat; after 20mins, increase the nitrogen pressure at a speed of 1ml / s Put the emulsified fluid into the reactor 6, and add 10L of sterilized pharmaceutical water at a temperature of 46°C to the reactor 6 in advance. During the process of injecting...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com