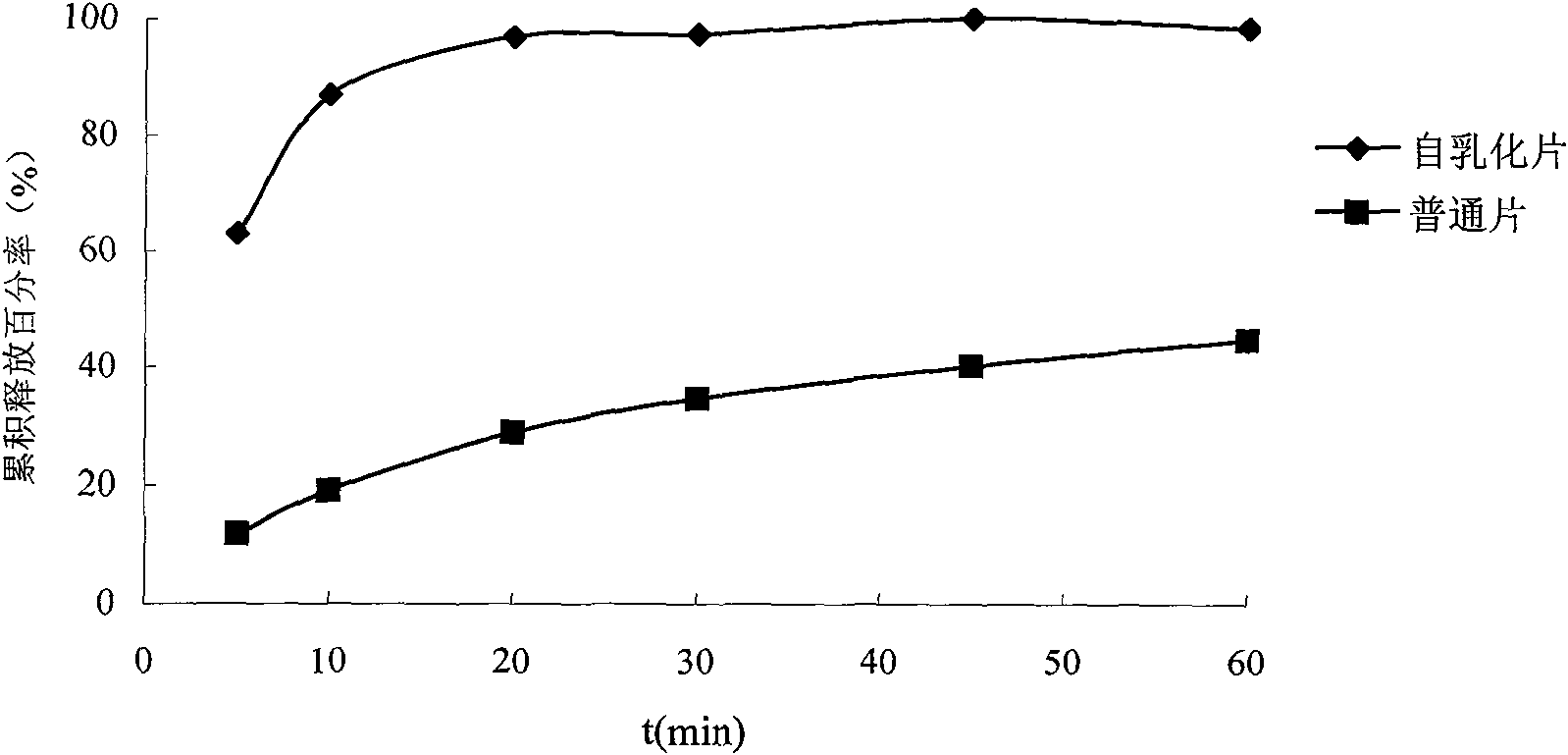
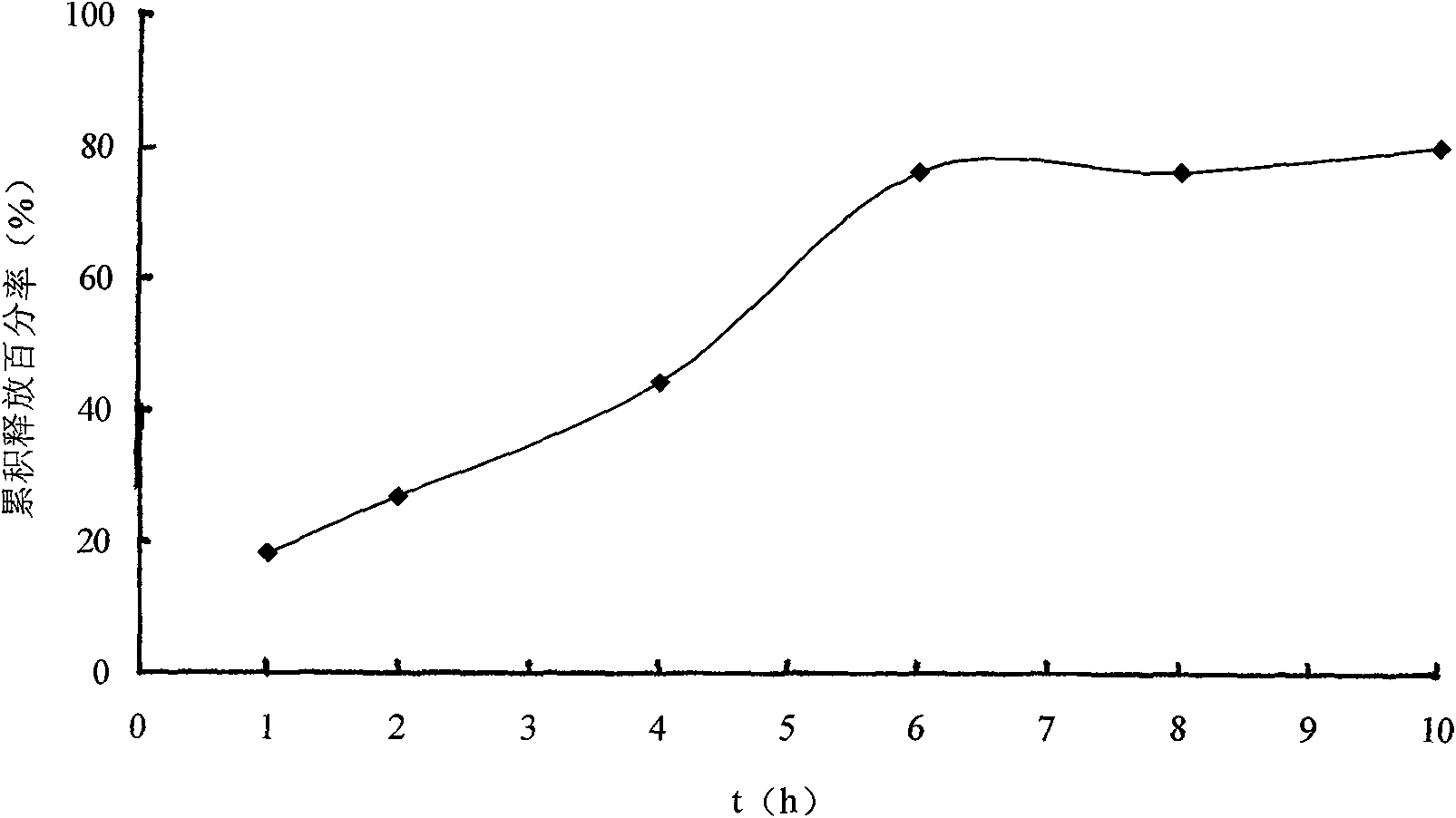
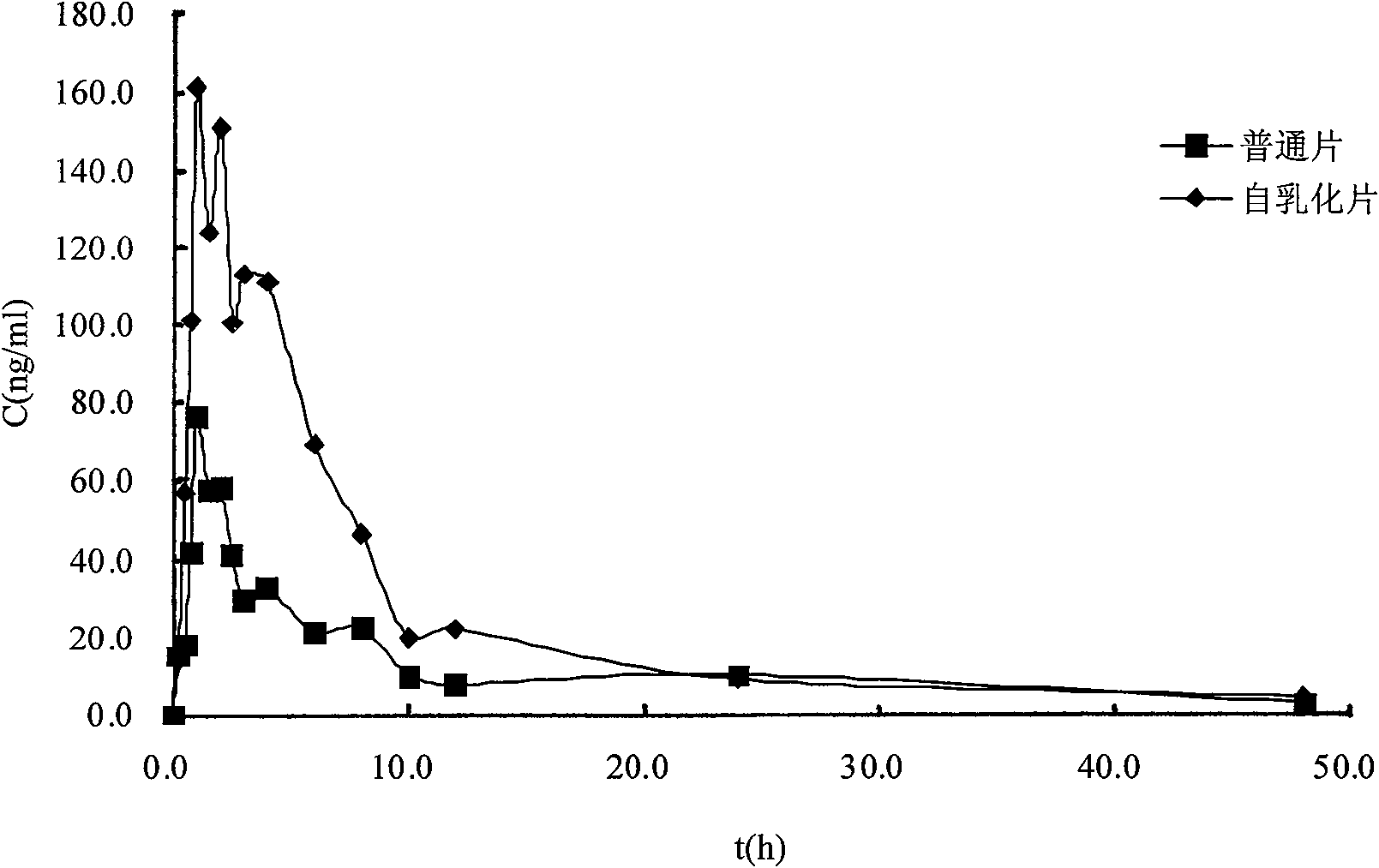
Solid self-emulsifying oral administration system of dihydropyridine calcium ion antagonist and preparation method thereof
The technology of calcium ion antagonist and dihydropyridine is applied in the solid self-emulsifying oral drug delivery system of dihydropyridine calcium ion antagonist and its preparation field, and can solve the problem of single dosage form, limitation of industrial production, and compatibility of capsule and shell. Drug precipitation and leakage, etc., to achieve the effect of facilitating dose distribution, improving bioavailability, and reducing fluctuations in blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 (drug-containing self-emulsifying system)
[0025] Prescription composition:
[0026] Nitrendipine 10%
[0027]Miglyol 812 30%
[0028] Cremophor RH40 40%
[0029] Tween 80 10%
[0030] Transcutol P 10%
[0031]
[0032] Preparation process: Take the prescribed amount of oil phase, surfactant and co-surfactant, mix evenly, add the prescribed amount of drug, and stir it with a magnetic force at 50°C to completely dissolve it to form a uniform drug-containing self-emulsifying system.
Embodiment 2
[0034] Prescription composition:
[0035] Felodipine 5%
[0036] Labrafil M 1944CS 70%
[0037] Cremophor RH40 20%
[0038] Transcutol P 5%
[0039]
[0040] The preparation process is the same as in Example 1.
Embodiment 3
[0042] Prescription composition:
[0043] Felodipine 5%
[0044] Labrafil M 1944CS 10%
[0045] Cremophor RH40 80%
[0046] Transcutol P 5%
[0047]
[0048] The preparation process is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com