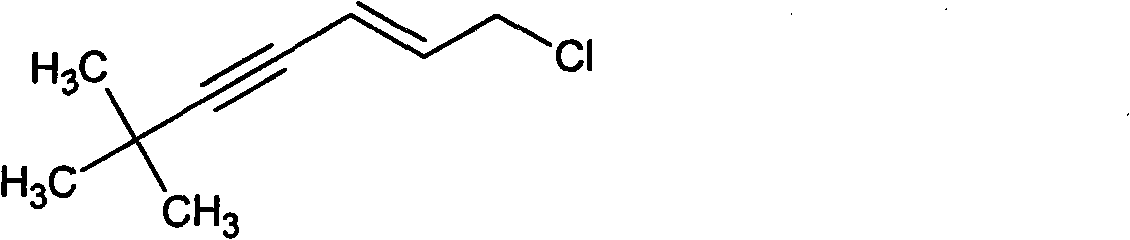
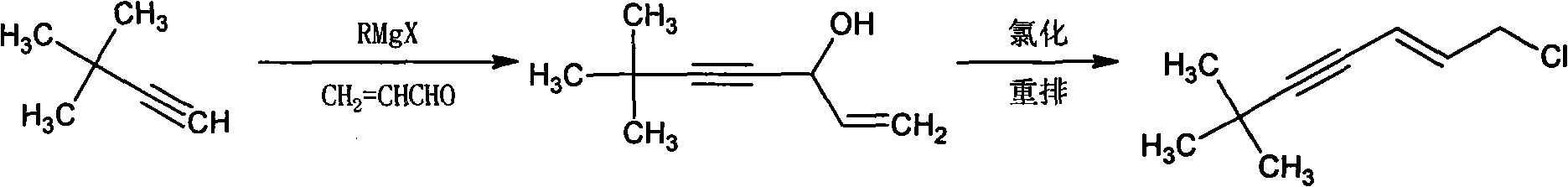
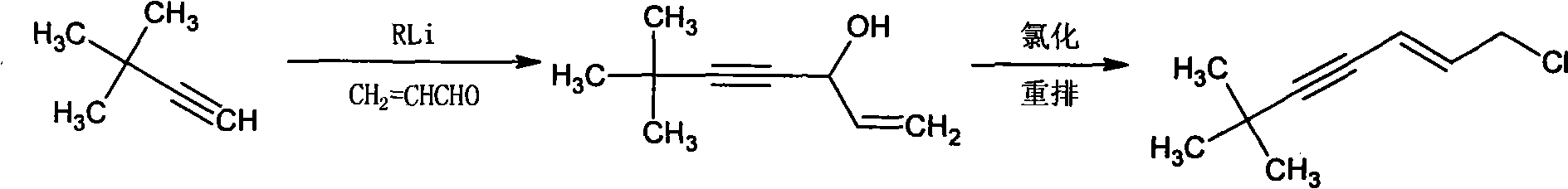
Method for synthesizing (E)-1-chlorine-6, 6-dimethyl-2-heptylene-4-alkyne
A synthesis method and dimethyl technology, applied in chemical instruments and methods, organic compound/hydride/coordination complex catalysts, organic chemistry, etc., can solve the problems of harsh reaction conditions, low product purity, and long synthesis route. , to achieve the effect of simple operation, high yield and few steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0022] Weigh 111 grams of trans-1,3-dichloropropene, 100 grams of tetrahydrofuran, and 204 grams of tri-n-butylamine, put them into the reaction flask, start stirring, add tetrakis (triphenylphosphine) palladium (0) 5.8 g, 0.96 g of cuprous iodide was added dropwise with 82 g of tert-butylacetylene, the rate of addition was controlled, and the temperature was maintained at 40°C. After the dropwise addition was completed, the reaction was incubated for 30 hours. After the reaction was completed, tetrahydrofuran was distilled off under reduced pressure. Add 200 grams of petroleum ether, stir for 20 minutes, wash three times with 8-12% dilute ammonia by weight, 100ml each time, dry, filter with suction, evaporate petroleum ether under reduced pressure to obtain a crude product.
[0023] Distill the crude product under reduced pressure and collect the fraction at 70-80°C (15mmHg) to obtain (E)-1-chloro-6,6-dimethyl-2-hepten-4-yne as a colorless to pale yellow transparent liquid ...
example 2
[0025] Weigh 111 grams of trans-1,3-dichloropropene, 100 grams of tetrahydrofuran, and 622 grams of tri-n-heptylamine, put them into the reaction flask, start stirring, add bis(triphenylphosphine)palladium(II) chloride 3.5 gram, cuprous iodide 0.96g, all the other are with example 1, obtain (E)-1-chloro-6,6-dimethyl-2-hepten-4-yne 138 grams; Product yield 88.1%.
Embodiment 3
[0027] Weigh 222 grams of trans-1,3-dichloropropene, 180 grams of tetrahydrofuran, and 303 grams of triethylamine, put them into the reaction flask, start stirring, add 5.1 grams of bis(tri-tert-butylphosphine) palladium and cuprous iodide 1.92 g, 164 g of tert-butylacetylene was added dropwise, the rate of addition was controlled, and the temperature was maintained at 40°C. After the dropwise addition was completed, the reaction was incubated for 20 hours. After the reaction was completed, tetrahydrofuran was distilled off under reduced pressure. Add 350 grams of petroleum ether, stir for 20 minutes, wash three times with 8-12% dilute ammonia by weight, 200ml each time, dry, filter with suction, evaporate petroleum ether under reduced pressure to obtain a crude product.
[0028] The crude product was distilled under reduced pressure, and the fraction at 70-80°C (15mmHg) was collected to obtain 270 g of (E)-1-chloro-6,6-dimethyl-2-hepten-4-yne; the product yield was 86.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com