Supermolecule type organic solar battery material and preparation method thereof
A solar cell and supramolecular technology, applied in circuits, photovoltaic power generation, electrical components, etc., can solve the problems of low mobility, low photoelectric conversion efficiency of organic solar cells, and inability to spontaneously dissociate, and achieve the effect of rapid transmission
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
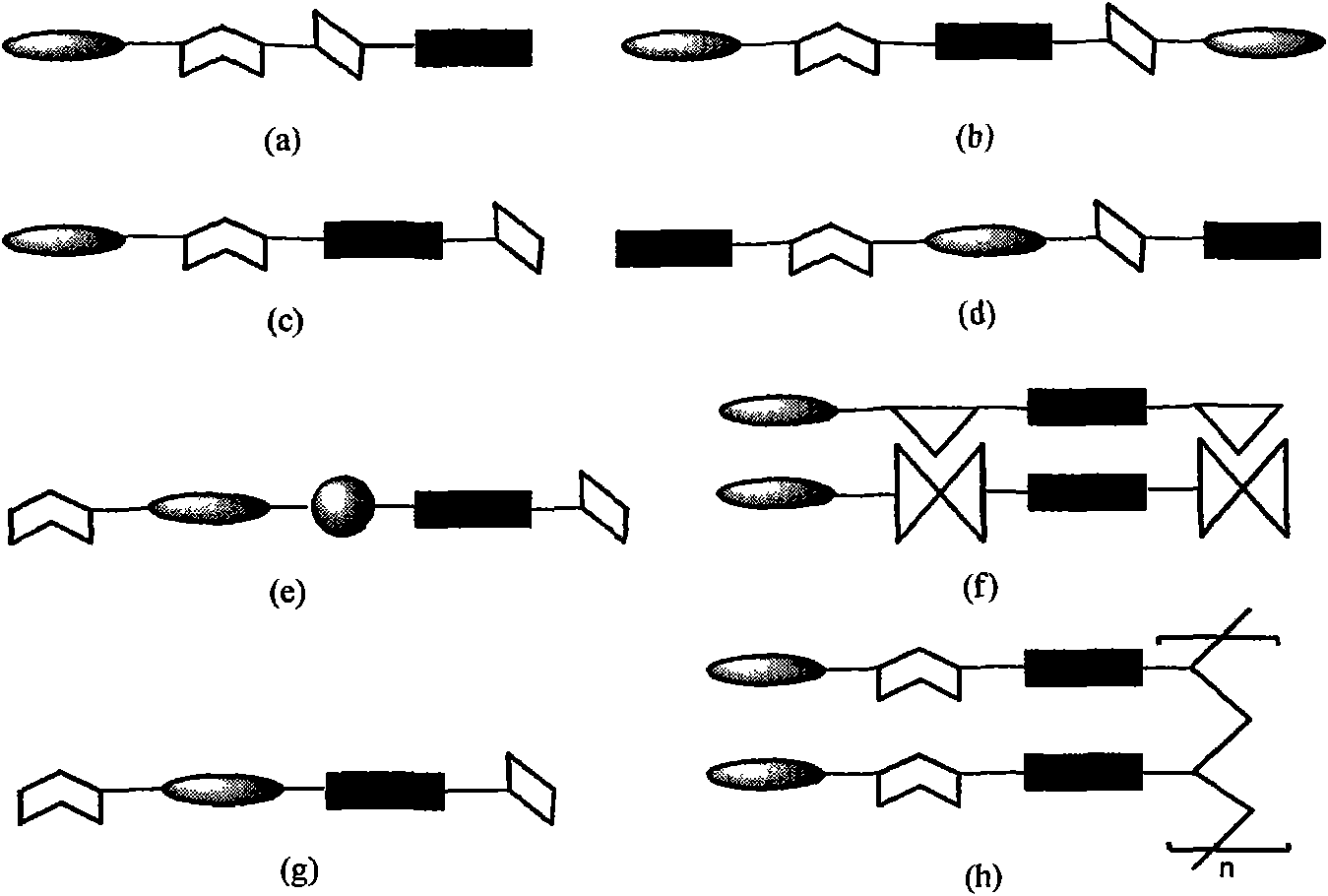
Image
Examples
Embodiment 1
[0051]
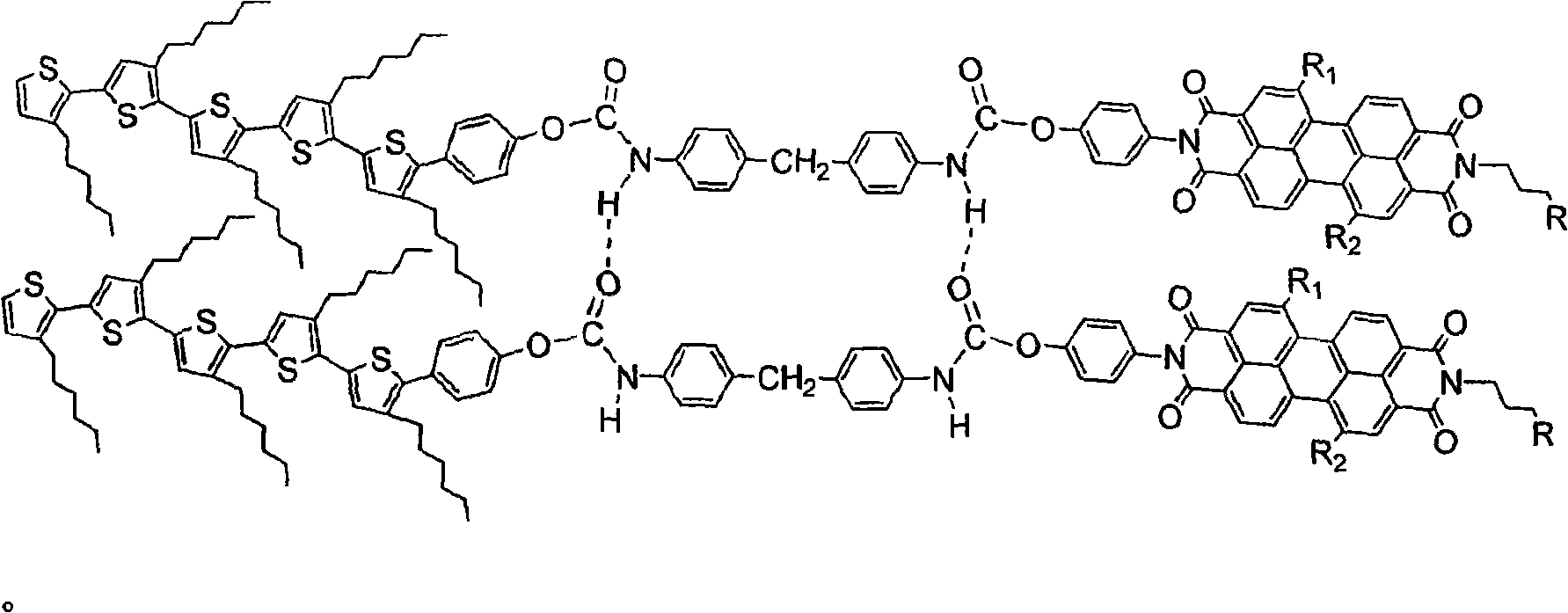
[0052] (1) Monobromination of pentahexylthiophene to prepare boric acid ester, and then suzuki reaction with p-bromophenol 1:1 to obtain phenol-substituted pentathiophene.
[0053] (2) Dissolving perylene diacid anhydride in solvents such as pyridine, imidazole or quinoline, adding dodecylamine under the catalysis of zinc acetate, reacting in a ratio of 1:1, and heating to reflux to prepare alkyl monoperylene anhydride. Then react with p-phenylenediamine in a ratio of 1:1, and heat to reflux to obtain perylene derivatives substituted with benzene and alkyl chains on nitrogen.
[0054] (3) Dissolve the product of reaction (1) in DMF solvent, heat to 100° C. and stir to reflux. The DMF solution of diisocyanate was slowly added dropwise and reacted for 4-5h. Add the product of reaction (2) and react according to 1:1 for 4-5 hours to obtain the target compound of pentathiophene perylene with phenol diisocyanate as the linking unit.
[0055] (4) Molecular self-assembl...
Embodiment 2
[0058]
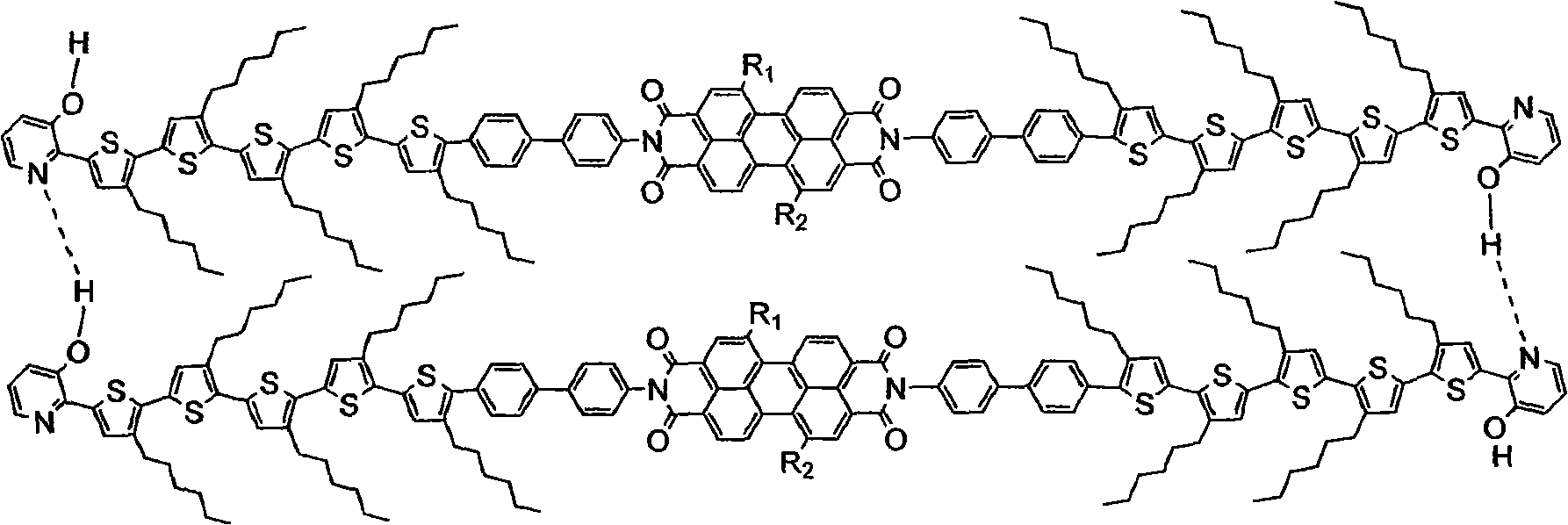
[0059] (1) Dibromination of pentahexylthiophene to prepare monoboronate, and then suzuki reaction with bromohydroxypyridine 1:1 to obtain monobromomonohydroxypyridine-capped pentathiophene.
[0060] (2) Boronate p-aminobromobenzene, and react with the product of reaction (1) 1:1 in a suzuki reaction to prepare monohydroxypyridine monoaminobenzene-terminated pentathiophene.
[0061] (3) dissolving perylene diacid anhydride in solvents such as pyridine, imidazole or quinoline, adding dodecyl amine under the catalysis of zinc acetate, and reacting in a 1:1 ratio to prepare alkyl monoperylene acid anhydride. Then react with the product of the reaction (2) at a ratio of 1:1, heat and reflux to obtain the target compound of pentathiophene perylene with hydroxypyridine as the assembly unit and benzene as the linking unit.
[0062] (4) Molecular self-assembly
[0063] Mix and stir 0.8mmol of the target product obtained after step (3), and equivalent EDC, 1-hydroxybenzotri...
Embodiment 3
[0065]
[0066] (1) Dibromination of pentahexylthiophene to prepare monoboronate, and then suzuki reaction with bromohydroxypyridine 1:1 to obtain monobromomonohydroxypyridine-capped pentathiophene.
[0067] (2) Boronate p-aminobromobenzene, and react with the product of reaction (1) 1:1 in a suzuki reaction to prepare monohydroxypyridine monoaminobenzene-terminated pentathiophene.
[0068] (3) Dissolving perylene diacid anhydride in a solvent such as pyridine, imidazole or quinoline, adding aminostyrene under the catalysis of zinc acetate, reacting at a ratio of 1:1, and heating to reflux for 2-3 hours to prepare styryl perylene anhydride. Alkyl perylene anhydride was prepared by reacting with 1:1. Reaction with the product of reaction (2) at a ratio of 1:1 to obtain the pentadthiophene perylene target compound with hydroxypyridine as the assembly unit and styrene and benzene ring as the linking unit.
[0069] (4) Add the product of reaction (3) to a solvent such as tolue...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com