Method for producing stannic chloride
A technology of tin tetrachloride and its production method, which is applied in the direction of tin chloride, tin halide, etc., can solve the problems of vicious cycle of harmful impurities, incomplete removal of harmful impurities in tin smelting, and high cost of raw materials, so as to increase the implementation of industrialization, Good versatility and the effect of increasing benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
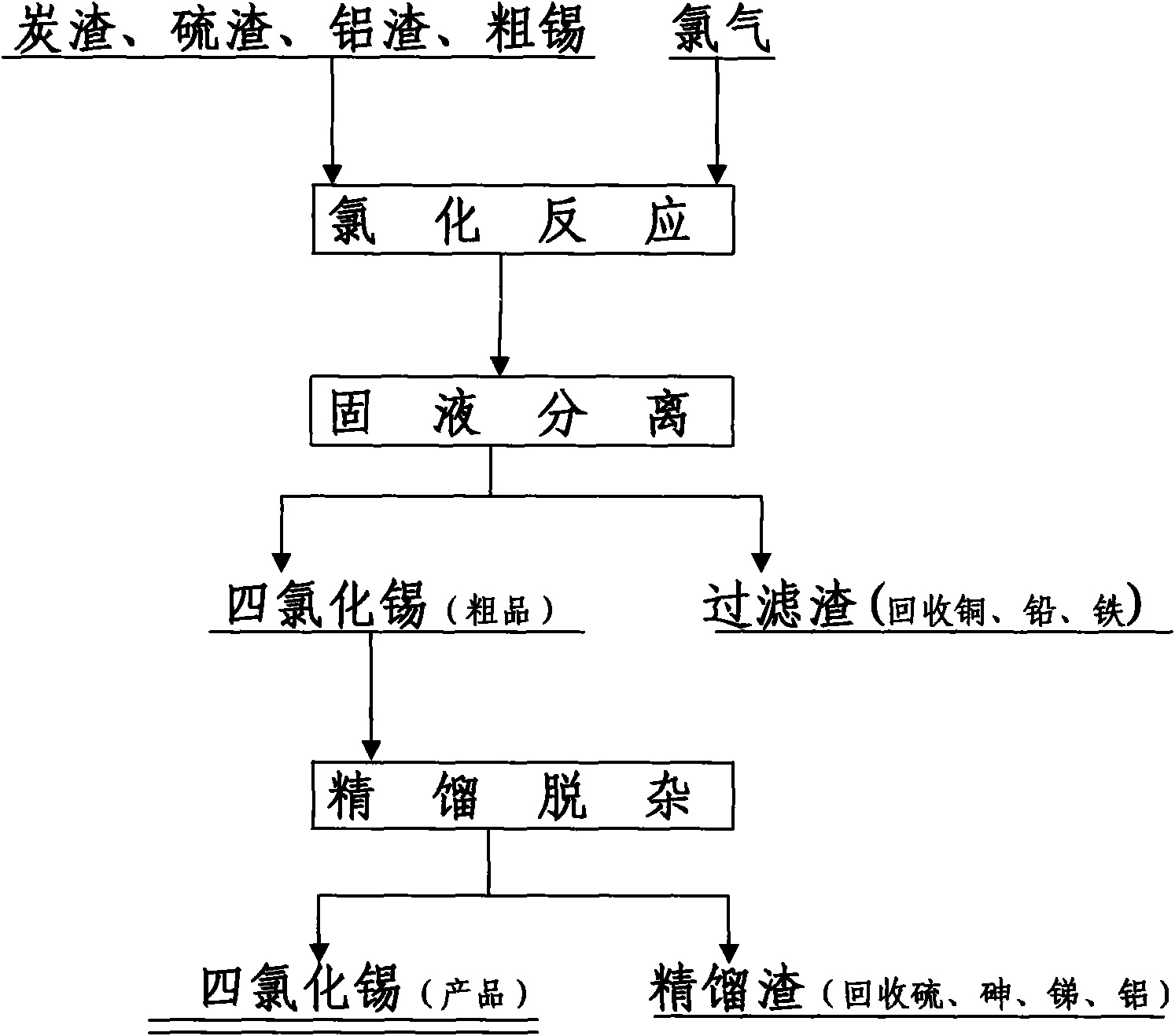
[0037] Add 1,000 kg of sulfur slag to a 1500 liter enamel reaction kettle, add 1000 kg of tin tetrachloride, seal the reaction kettle, and slowly introduce chlorine gas with slow stirring. Add cooling water to the jacket of the enamel reaction kettle, adjust the flow of cooling water, adjust the flow of chlorine, and control the reaction temperature of the materials in the kettle at 80-90°C, and react for 7 hours. Stop the introduction of chlorine gas and stop stirring. The conversion rate of tin in the slag into tin tetrachloride (tin leaching rate) is greater than 90%. Filtration, recover cuprous chloride and lead chloride from the filter cake produced by filtration. The filtrate is crude tin tetrachloride, which is added to the rectification tower for rectification and impurity removal to remove antimony trichloride and arsenic trichloride And sulfur. Antimony is recovered from antimony trichloride, arsenic is recovered from arsenic trichloride, and sulfur is returned to th...
example 2
[0039] Add 400kg of charcoal residue, 300kg of sulfur residue, 300kg of aluminum residue into a 1500-liter enamel reactor, add 1500kg of tin tetrachloride, seal the reactor, and slowly introduce chlorine gas with slow stirring. Add cooling water to the jacket of the enamel reactor, adjust the flow of the cooling water, adjust the flow of chlorine, and control the reaction temperature of the materials in the kettle at 100-110°C, and react for 5 hours. Stop the introduction of chlorine gas and stop stirring. The conversion rate of tin in the slag into tin tetrachloride (tin leaching rate) is greater than 90%. Filtration, recover cuprous chloride and lead chloride from the filter cake produced by filtration. The filtrate is crude tin tetrachloride, which is added to the rectification tower for rectification and impurity removal to remove antimony trichloride and aluminum trichloride , Arsenic trichloride and sulfur. Antimony is recovered from antimony trichloride, aluminum is rec...
example 3
[0041] Add 1000 kg of aluminum slag into a 1500 liter tubular reactor, add 500 kg of tin tetrachloride, seal the reactor, and slowly introduce chlorine gas with slow stirring. Add cooling water to the jacket of the reactor, adjust the flow of cooling water, adjust the flow of chlorine, and control the reaction temperature of the materials in the reactor at 60-70°C, and react for 9 hours. Stop the introduction of chlorine gas and stop stirring. The conversion rate of tin in the slag into tin tetrachloride (tin leaching rate) is greater than 90%. Filtration, recover cuprous chloride and lead chloride from the filter cake produced by filtration. The filtrate is crude tin tetrachloride, which is added to the rectification tower for rectification and impurity removal to remove antimony trichloride and aluminum trichloride , Arsenic trichloride. Antimony is recovered from antimony trichloride, aluminum is recovered from aluminum trichloride, and arsenic is recovered from arsenic tri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com