Aliphatic polycarbonate with high molecular weight and preparation method thereof
A polycarbonate, high molecular weight technology, applied in the field of aliphatic polycarbonate and its preparation, can solve the problems of high toxicity of phosgene, decarbonation, poor production conditions, etc., avoid catalyst separation process, improve heat resistance, Overcome costly effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1, preparation aliphatic polycarbonate
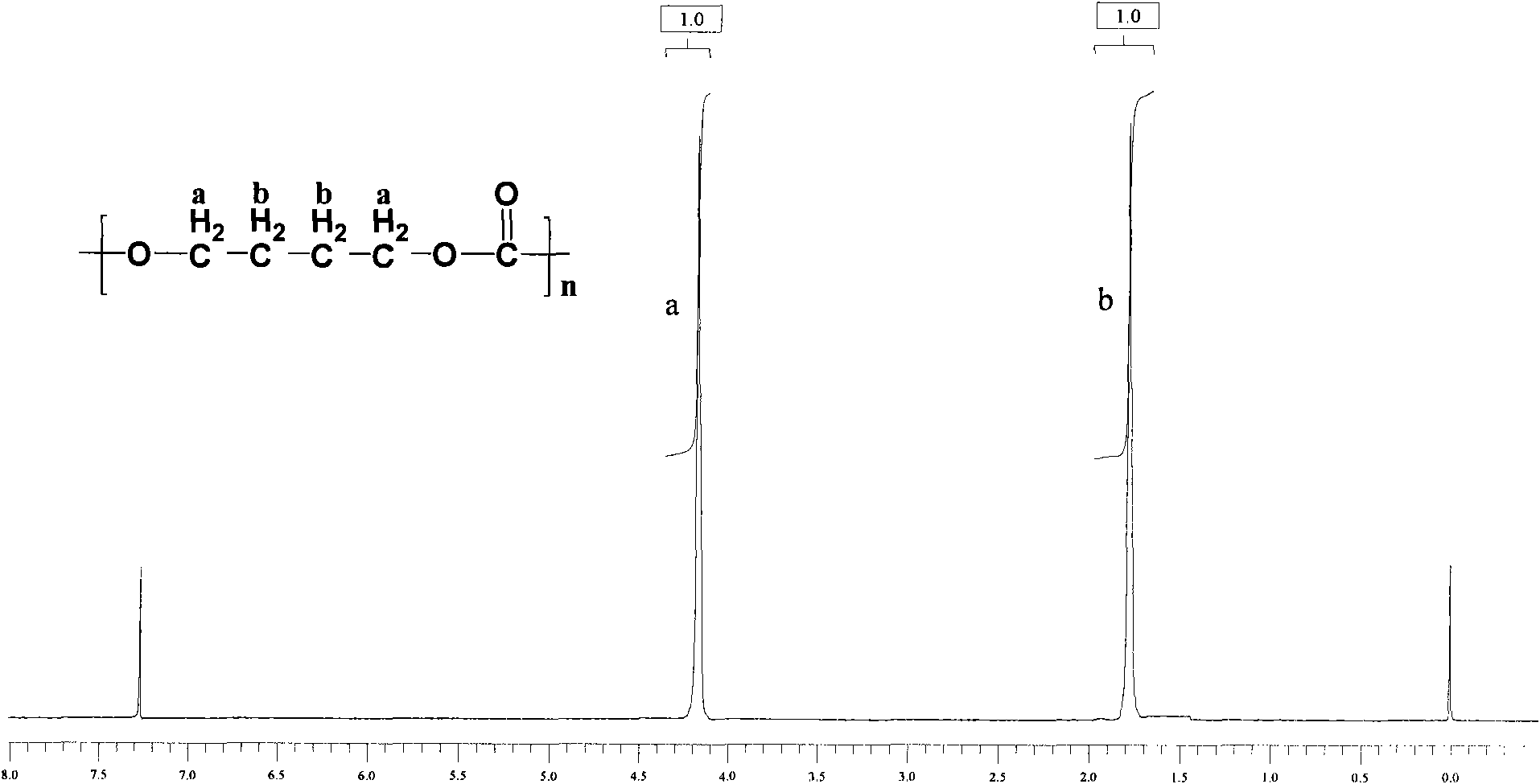
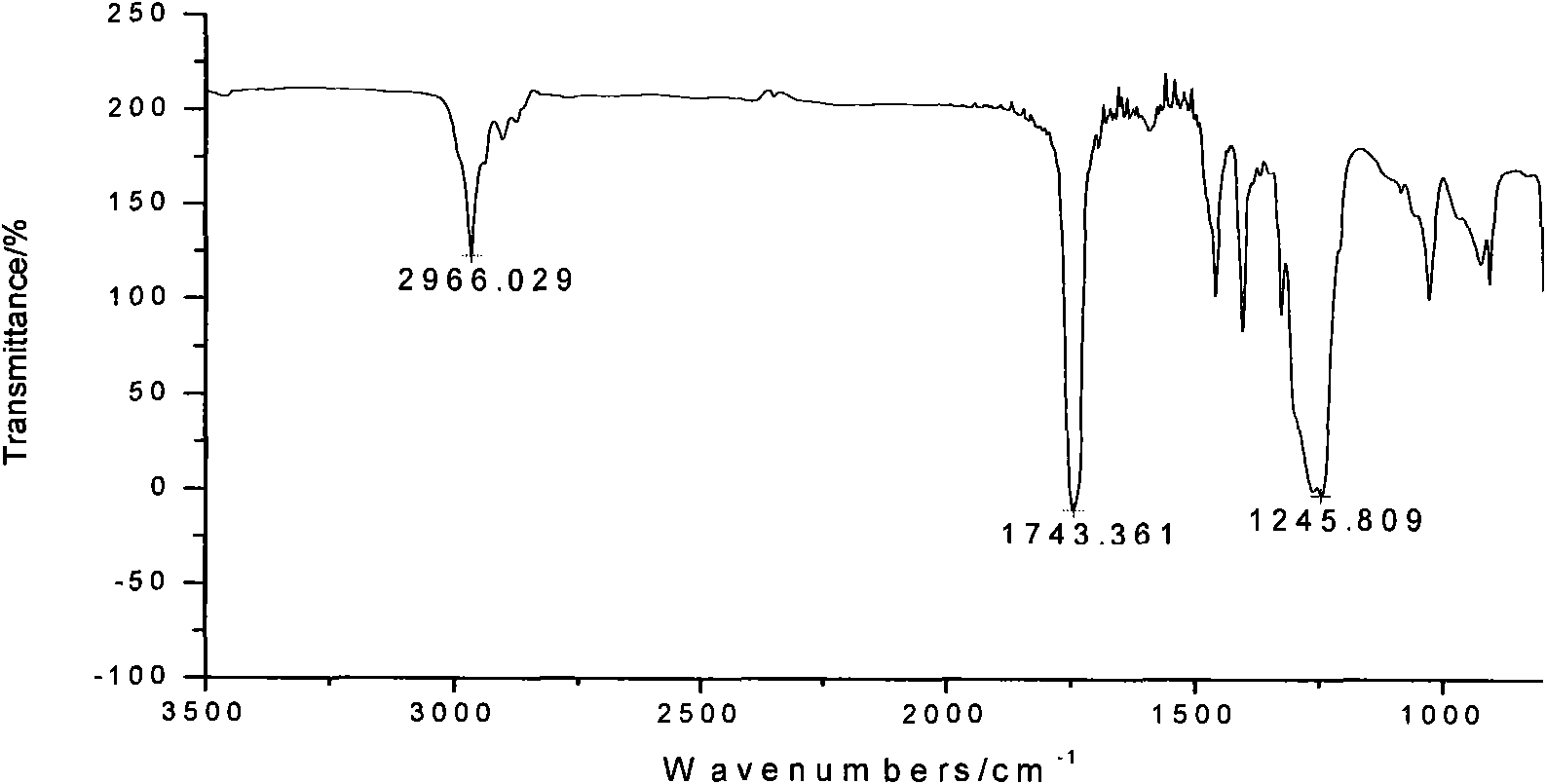
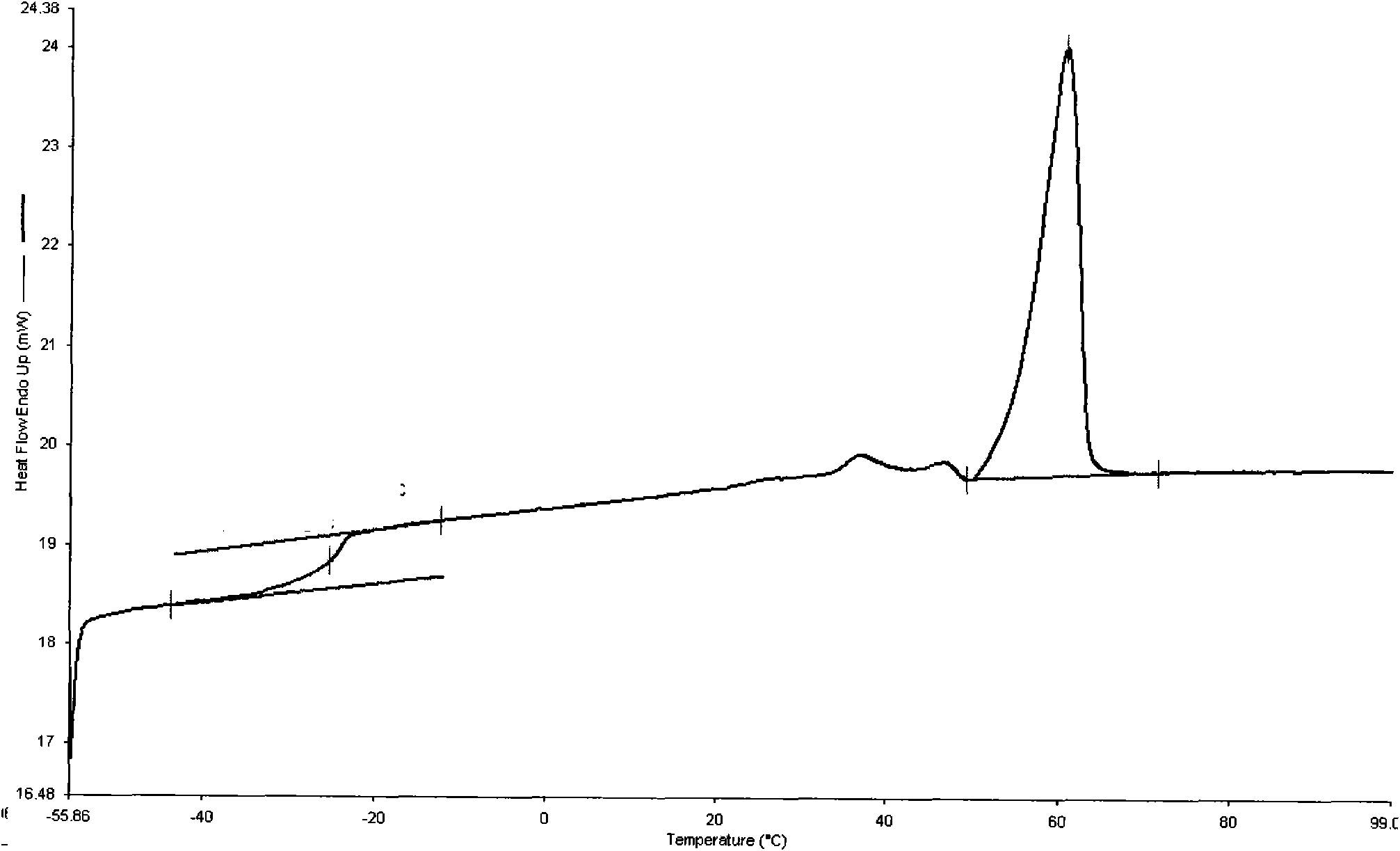
[0030] In a 250ml glass flask, add 30g dimethyl carbonate, 60g 1,4-butanediol and 0.25ml tetrabutyl titanate (the molar ratio of dimethyl carbonate to 1,4-butanediol 1:2), start the reaction with stirring at 120°C, gradually raise the temperature to 225°C, each temperature stage must react completely before heating up, that is, no methanol will be distilled out, until all the methanol produced and unreacted dimethyl carbonate All esters were distilled off. Add 0.2ml of tetrabutyl titanate, and polycondense at 240°C for 5 hours under the pressure of less than 200Pa to finally obtain 25g of polycarbonate A with an intrinsic viscosity of 0.86dl / g and a number average molecular weight of 24300. The polycarbonate 1 H-NMR spectrum as figure 1 As shown, the FT-IR spectrum is as figure 2 shown. Depend on figure 1 It can be seen that the structure of the polymer is correct; figure 2 Medium, 2966cm -1 、1743cm -1 、7245...
Embodiment 2
[0031] Embodiment 2, prepare aliphatic polycarbonate
[0032]In a 250ml glass flask, add 90g dimethyl carbonate, 30g 1,4-butanediol and 0.005g potassium carbonate under nitrogen atmosphere (the molar ratio of dimethyl carbonate to 1,4-butanediol is 3:1 ), start the reaction with stirring at 120°C, and then gradually increase the temperature to 225°C (each temperature stage must react completely before heating up, that is, no methanol will be distilled), until all the methanol produced and unreacted dimethyl carbonate are completely Evaporate; add 0.15g of lithium carbonate, polycondensate at 240°C for 5 hours under a pressure lower than 200Pa, and finally obtain 30g of polycarbonate B with an intrinsic viscosity of 1.51dl / g and a number average molecular weight of 55,400.
Embodiment 3
[0033] Embodiment 3, preparation aliphatic polycarbonate
[0034] In a 250ml glass flask, add 55g dimethyl carbonate, 52g 1,5-pentanediol and 0.002g of manganese acetate (the mol ratio of dimethyl carbonate to 1,5-pentanediol is 1: 1.22), start the reaction with stirring at 120°C, and then gradually increase the temperature to 225°C (each temperature stage must react completely before heating up, that is, no methanol will be distilled out), until all the methanol produced and unreacted dimethyl carbonate All was evaporated; 0.2g of lithium carbonate was added, polycondensed for 6 hours at 250°C and a pressure lower than 200Pa, and finally 58g of polycarbonate C was obtained, with an intrinsic viscosity of 1.08dl / g and a number average molecular weight of 35,000.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com