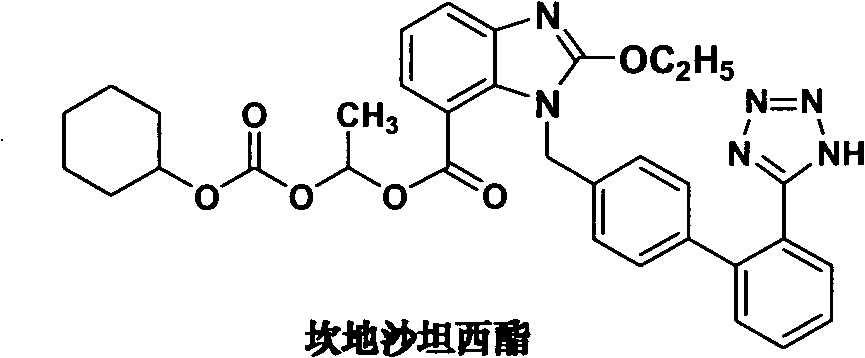
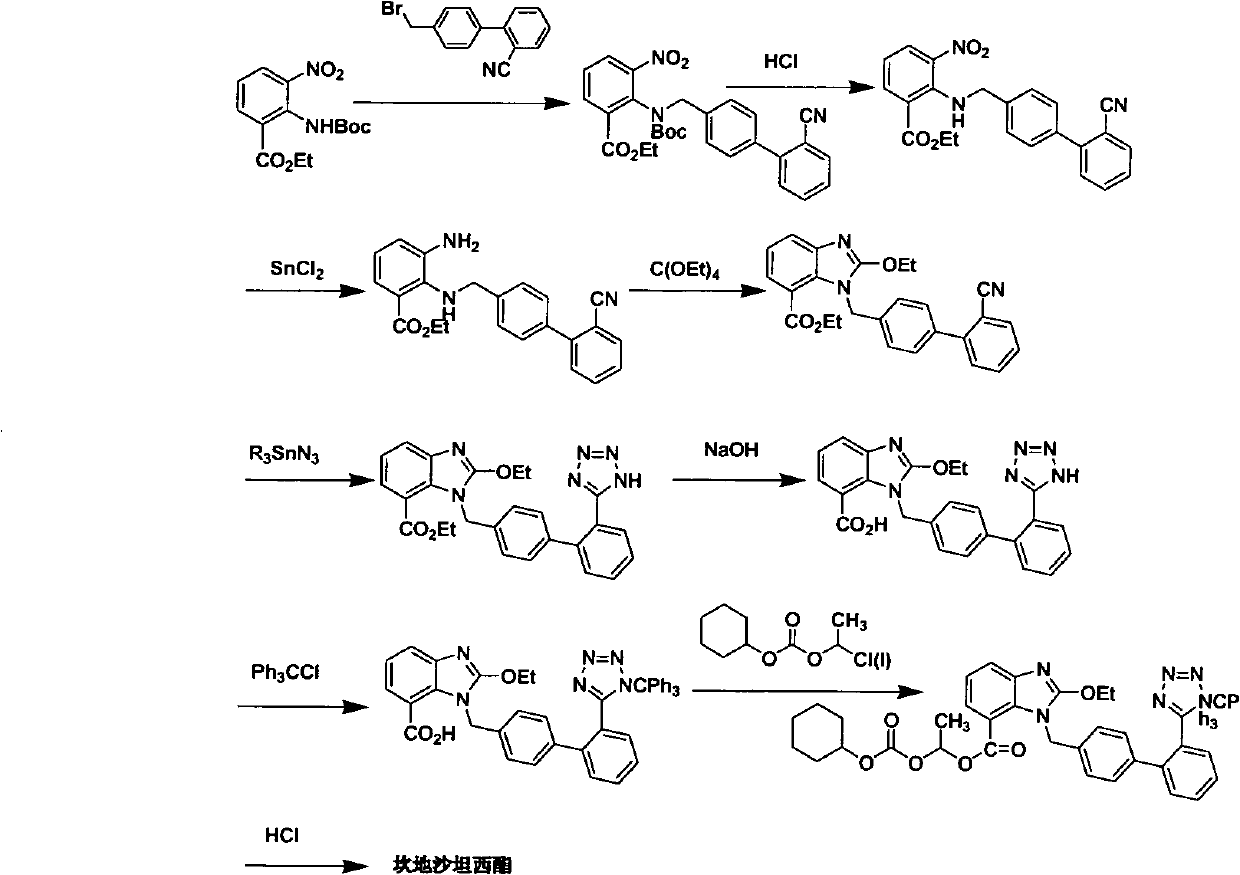
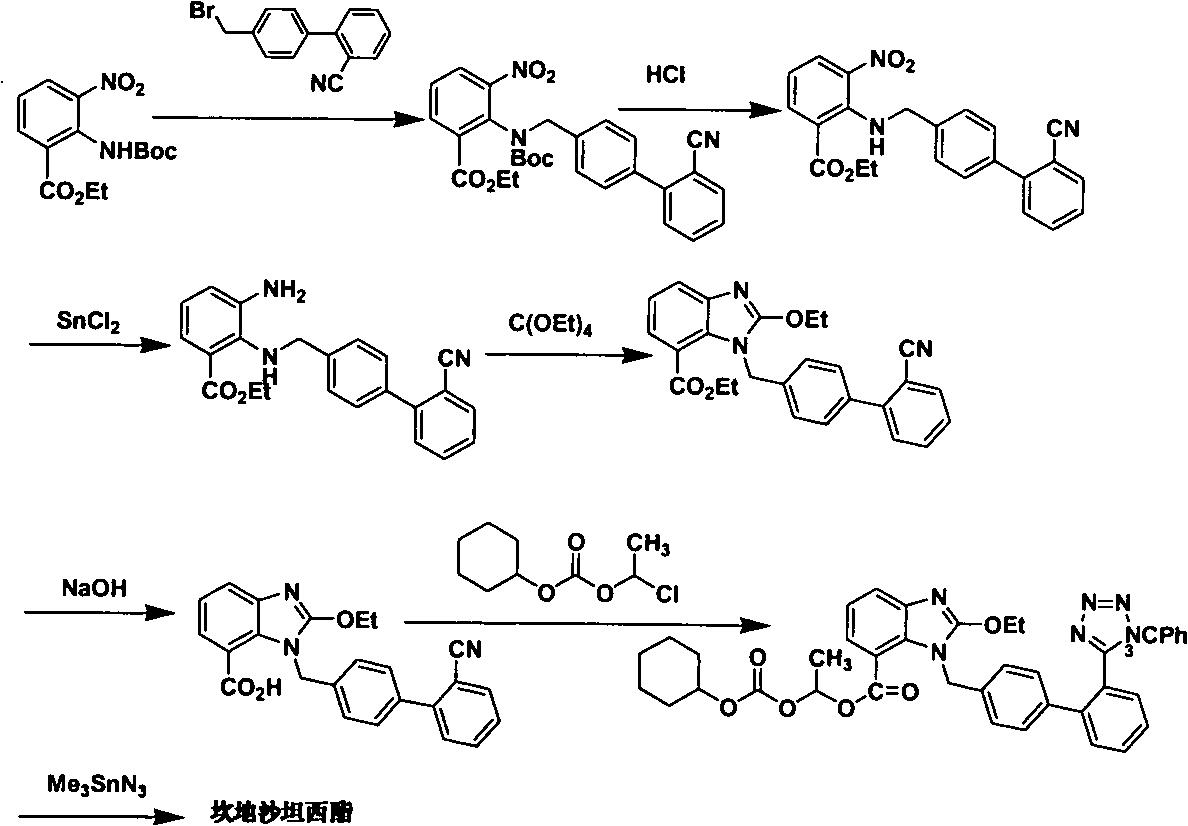
The method for making candixatan ester and intermediates thereof
A compound, the technology of methoxycarbonyl, applied in the field of preparation of candesartan cilexetil and its intermediates, can solve the problem of low total yield, and achieve the effect of simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1: 2-[N-(tert-butoxycarbonyl)-[4-[2'-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl]amino]-3 - Preparation of ethyl nitrobenzoate (formula V, R1 = tert-butoxycarbonyl, R2 = trityl)
[0072] Ethyl 2-tert-butoxycarbonylamino-3-nitrobenzoate (1.06g, 3.41mmol), 2-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl bromide (1.91mg, 3.42mmol), potassium carbonate (0.7g, 5.07mmol) and dry DMF (15ml) were mixed and fully reacted at 30°C-120°C (60°C for 2 hours was specifically selected). After cooling, pour into ice water (30ml), stir for half an hour, extract with ethyl acetate (20ml×3), wash the organic phase with water (20ml×2), wash with saturated brine (20ml), and dry over anhydrous sodium sulfate. Concentration gave a yellow oil, which was recrystallized from acetone-water to give 2-[N-(tert-butoxycarbonyl)-[4-[2′-(1-trityl-1H-tetrazolium- 5-yl)biphenylmethyl]amino]-3-nitrobenzoic acid ethyl ester (V, R1 = tert-butoxycarbonyl, R2 = trityl) (2.3 g, 90%).
Embodiment 2
[0073] Example 2: 2-[N-(tert-butoxycarbonyl)-[4-[2'-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl]amino]-3 - Preparation of nitrobenzoic acid (formula IV, R1 = tert-butoxycarbonyl, R2 = trityl)
[0074] The compound (2.01g, 2.56mmol) represented by the formula V (R1 = tert-butoxycarbonyl, R2 = trityl) was dissolved in THF (5ml) / water (1ml) solution, and sodium hydroxide (113mg, 2.83 mmol), fully react under the condition of 40°C-70°C (specifically choose 60°C for 24 hours). The THF was evaporated under reduced pressure, the residue was dissolved in water (20ml), and the pH value was adjusted to 5 with 1N hydrochloric acid. A large amount of white precipitates precipitated out. The precipitates were collected and dried to obtain 2-[N-(tert-butoxycarbonyl)- [4-[2′-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl]amino]-3-nitrobenzoic acid (IV, R1=tert-butoxycarbonyl, R2 =trityl) (1.85 g, 98%). 1 H NMR (CDCl 3 -d6, 300MHz), δ1.6 (2s, 9H), 4.5 (d+d, 2H), 6.8-7.9 (m, 26H).
Embodiment 3
[0075] Example 3 (±) 2-[N-(tert-butoxycarbonyl)-[4-[2′-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl]amino] - Preparation of 3-nitrobenzoic acid-1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester (formula III, R1=tert-butoxycarbonyl, R2=trityl)
[0076] Compound (1.85g, 2.4mmol), 1-chloroethylcyclohexyl carbonate (700mg, 3.4mmol), potassium carbonate (500mg, 3.4mmol) and dry DMF (10ml) were mixed, and the reaction was complete under the condition of 20°C-60°C (specifically choose 50°C for 4 hours). After cooling, pour into ice water (30ml), stir for half an hour, extract with ethyl acetate (20ml×3), wash the organic phase with water (20ml×2), wash with saturated brine (20ml), and dry over anhydrous sodium sulfate. (±) 2-[N-(tert-butoxycarbonyl)-[4-[2′-(1-trityl-1H-tetrazol-5-yl)biphenylmethyl) in white semi-solid ]amino]-3-nitrobenzoic acid-1-[[(cyclohexyloxy)carbonyl]oxy]ethyl ester (III, R1 = tert-butoxycarbonyl, R2 = trityl) (1.78g, 80 %). 1 H NMR (DMSO-d6, 300MHz), δ1.1-2.0 (m, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com