Plating method for preventing hydrogen bubbles of copper-tin alloy plating layer
A technology of copper-tin alloy and coating, which is applied in the field of pulse electroplating technology to prevent hydrogen bubbling of copper-tin alloy coating, can solve the problems of affecting material performance, reducing material toughness, and no one has raised it, achieving strong applicability, low cost, The effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
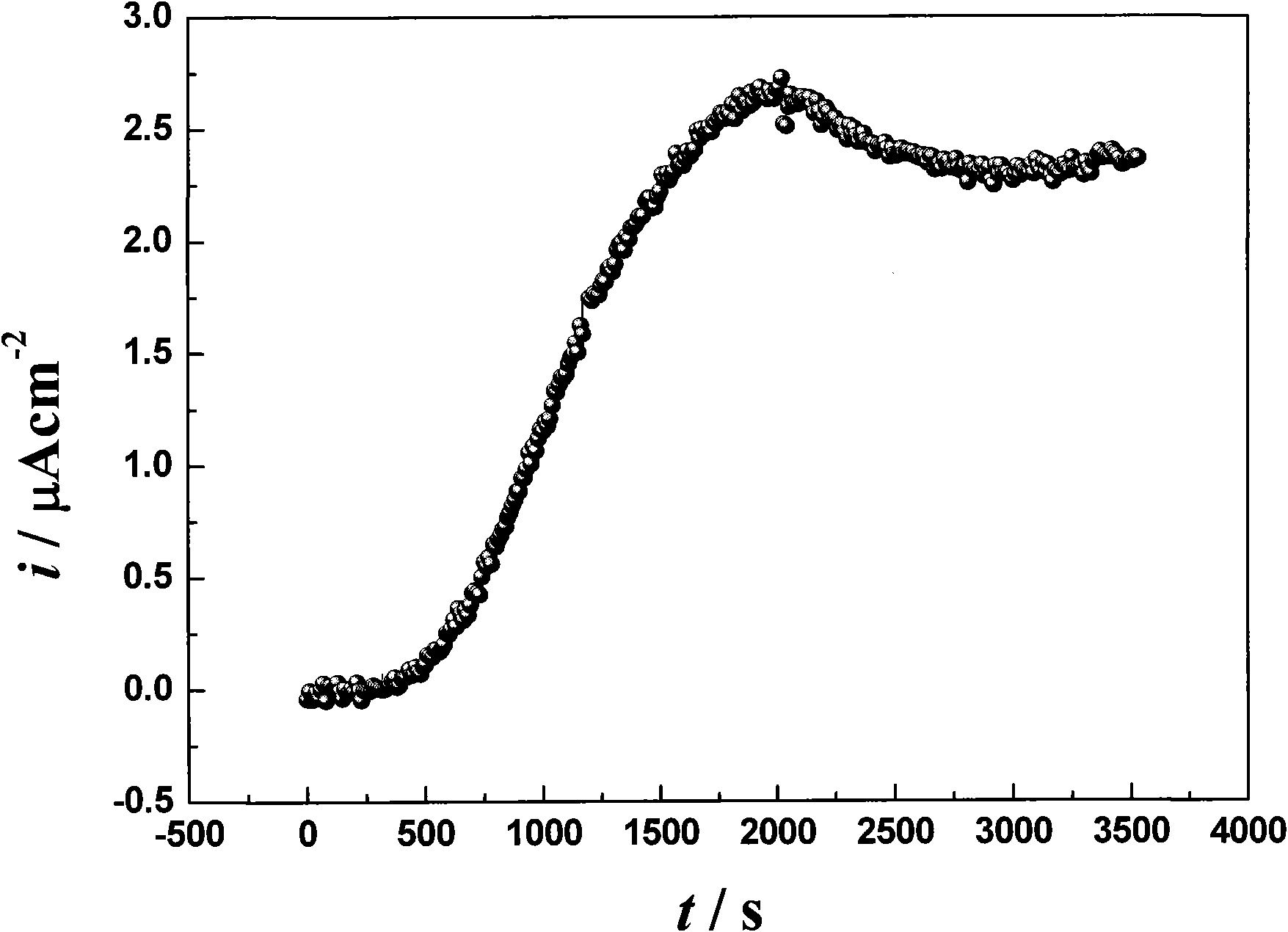
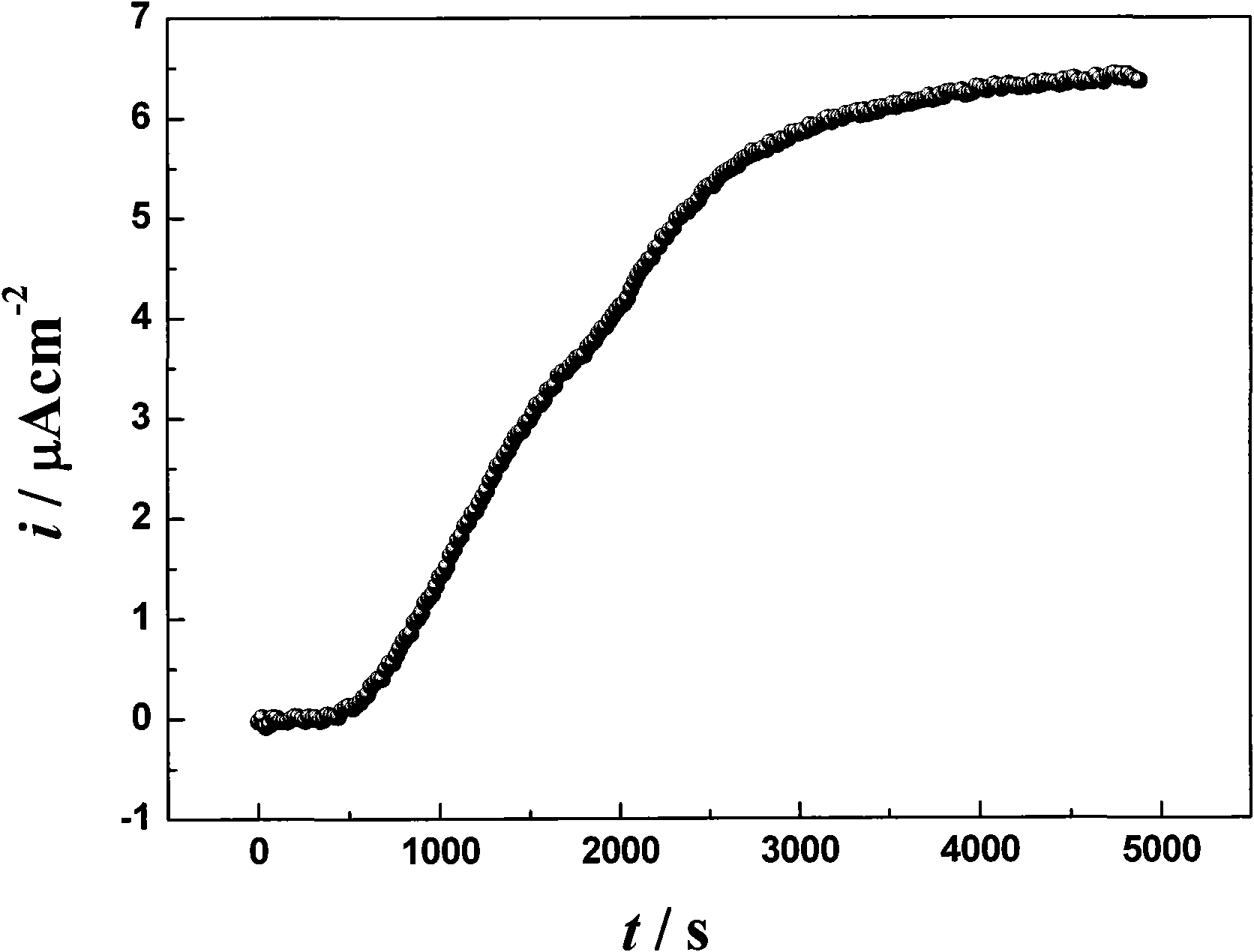
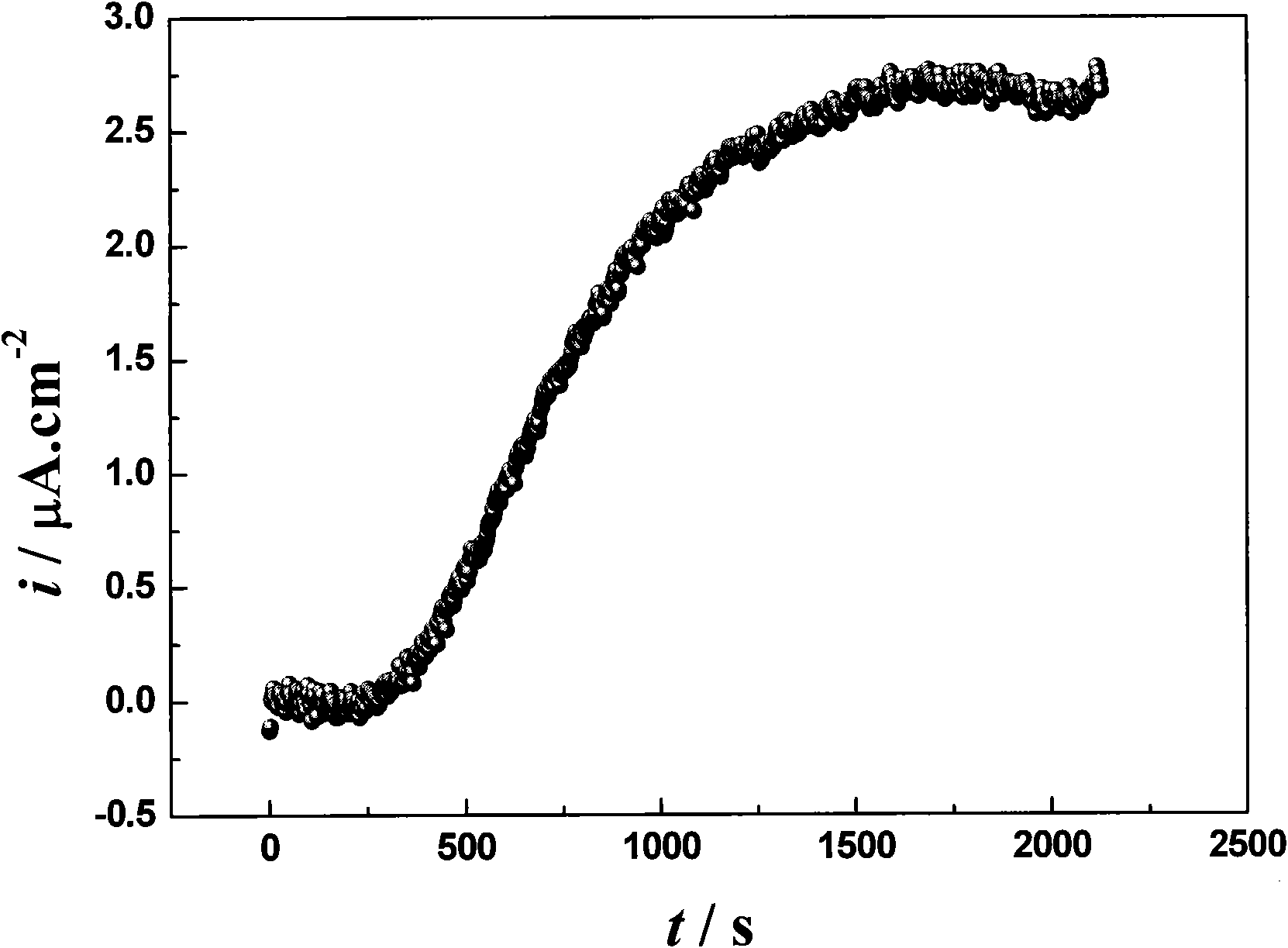
Image
Examples
Embodiment 1
[0026] 1. Preparation of copper-tin alloy coating by pulse electroplating technology
[0027] Electrolytic deposition equipment: pulsed electrolytic deposition equipment
[0028] Requirements for the electrolyte used in electrolytic deposition: the copper-tin alloy electroplating solution is the main salt is potassium pyrophosphate (K 4 P 2 o 7 ) 240-280g / L, copper pyrophosphate (Cu 2 P 2 o 7 ·3H 2 O)34-47g / L and stannous pyrophosphate (Sn 2 P 2 o 7 ) 2.6-4.3g / L pyrophosphate low-tin bronze electrolyte, pH 8-9; strictly control the content of heavy metal impurities in the electrolyte, the water used for distributing the electrolyte is high-purity deionized water, and the pH of the electrolyte is 8~9.
[0029] Cathode and anode material requirements: the anode is a high-purity 99.99% pure copper plate, and the cathode is a 27SiMn steel plate after degreasing treatment.
[0030] 2. Electrolytic process parameters: square wave pulse electroplating. Pulse average curre...
Embodiment 2
[0035] 1. Preparation of copper-tin alloy coating by pulse electroplating technology
[0036] Electrolytic deposition equipment: pulsed electrolytic deposition equipment
[0037] Requirements for the electrolyte used in electrolytic deposition: the copper-tin alloy electroplating solution is the main salt is potassium pyrophosphate (K 4 P 2 o 7 ) 240-280g / L, copper pyrophosphate (Cu 2 P 2 o 7 ·3H 2O)34-47g / L and stannous pyrophosphate (Sn 2 P 2 o 7 ) 2.6-4.3g / L pyrophosphate low-tin bronze electrolyte, pH 8-9; strictly control the content of heavy metal impurities in the electrolyte, the water used for distributing the electrolyte is high-purity deionized water, and the pH of the electrolyte is 8~9.
[0038] Cathode and anode material requirements: the anode is a high-purity 99.99% pure copper plate, and the cathode is a 27SiMn steel plate after degreasing treatment.
[0039] 2. Electrolytic process parameters: square wave pulse electroplating. Pulse average curren...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com