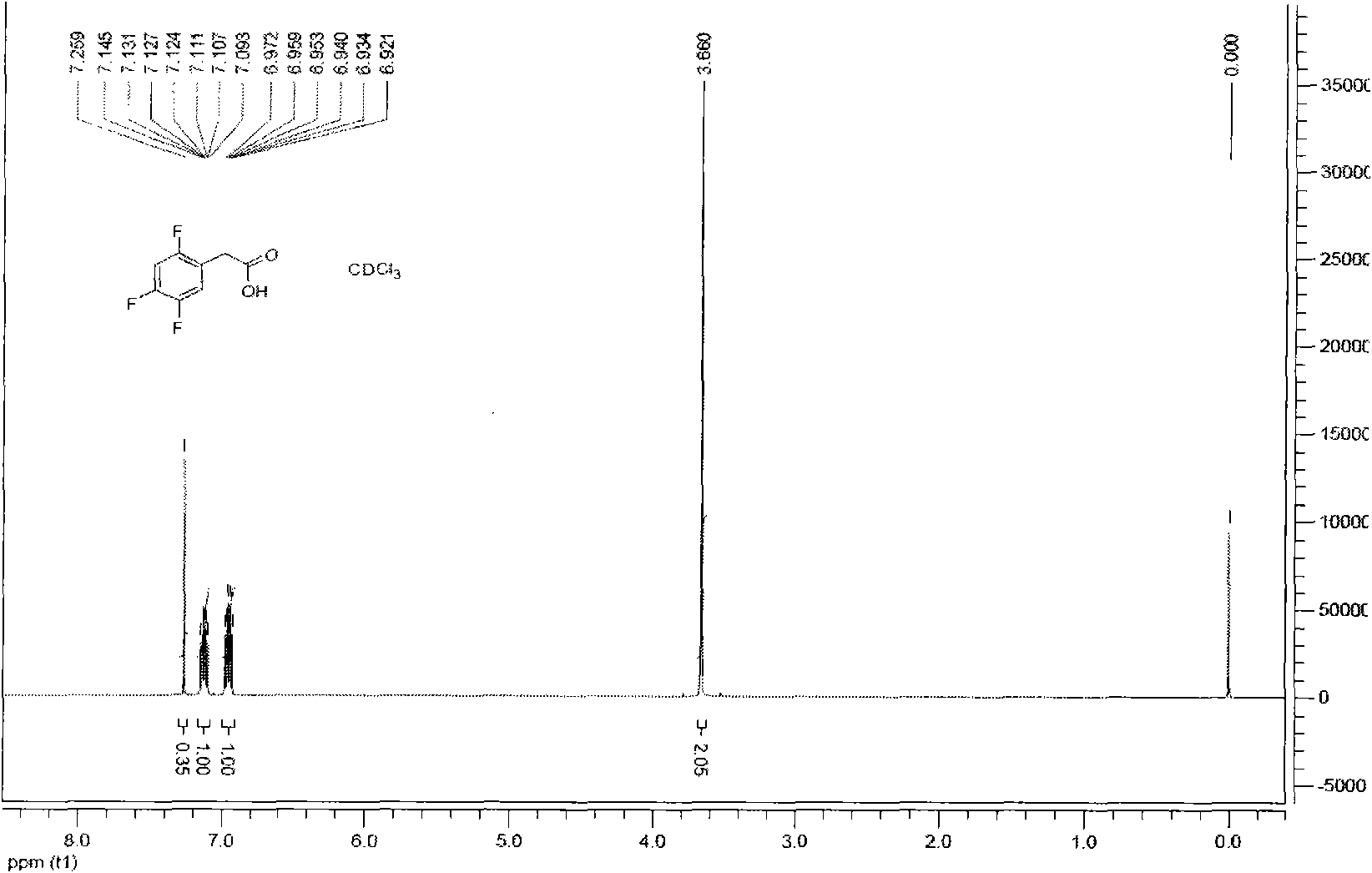Method for preparing 2, 4, 5-trifluoro-phenylacetic-acid
A technology of trifluorophenylacetic acid and trifluorobenzyl chloride, applied in 2 fields, can solve the problems of unsuitable industrialization, difficult operation, poor environmental friendliness, etc., and achieve the effects of mild reaction conditions, strong operability, and good product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: the preparation of 2,4,5-trifluorobenzyl chloride
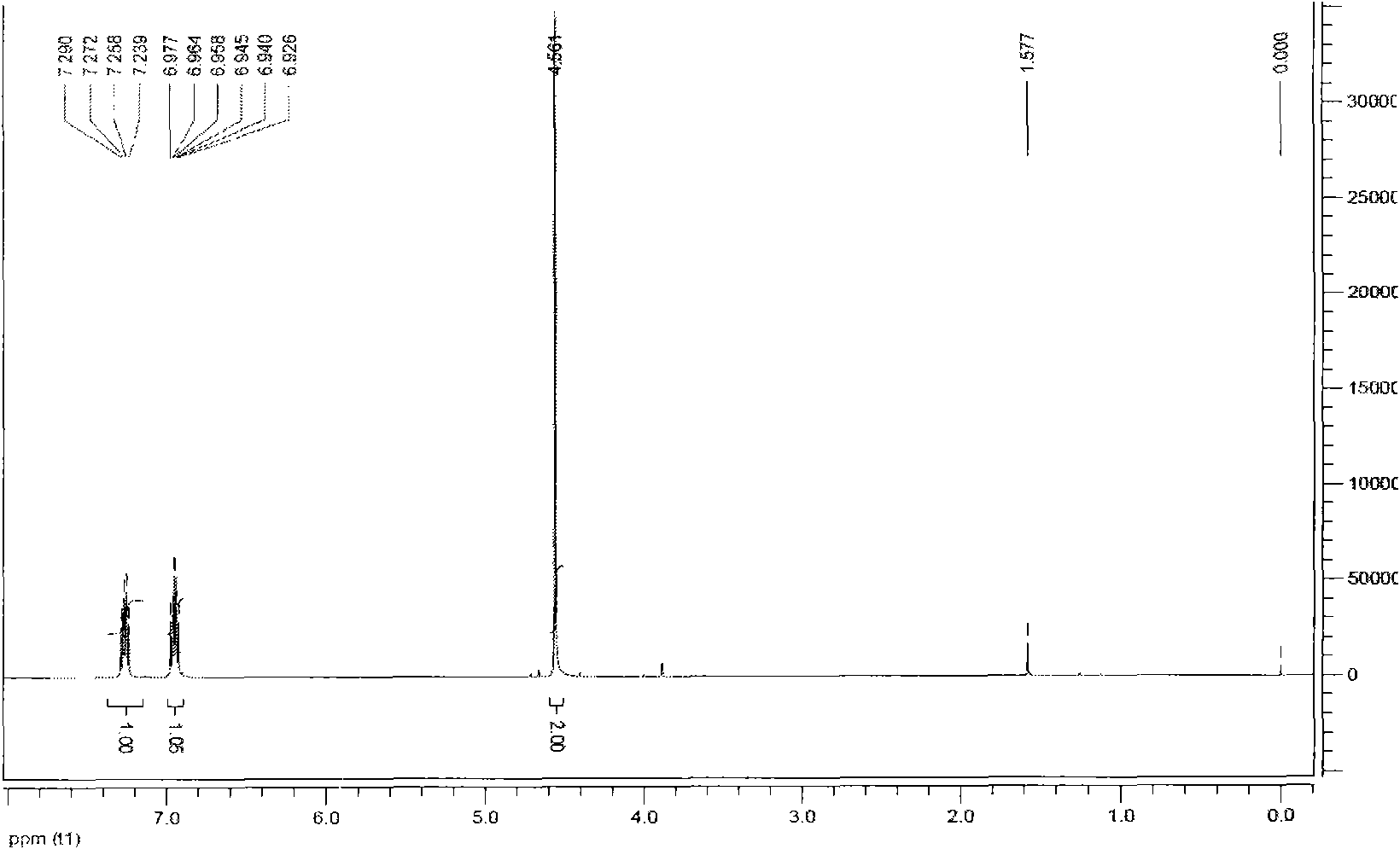
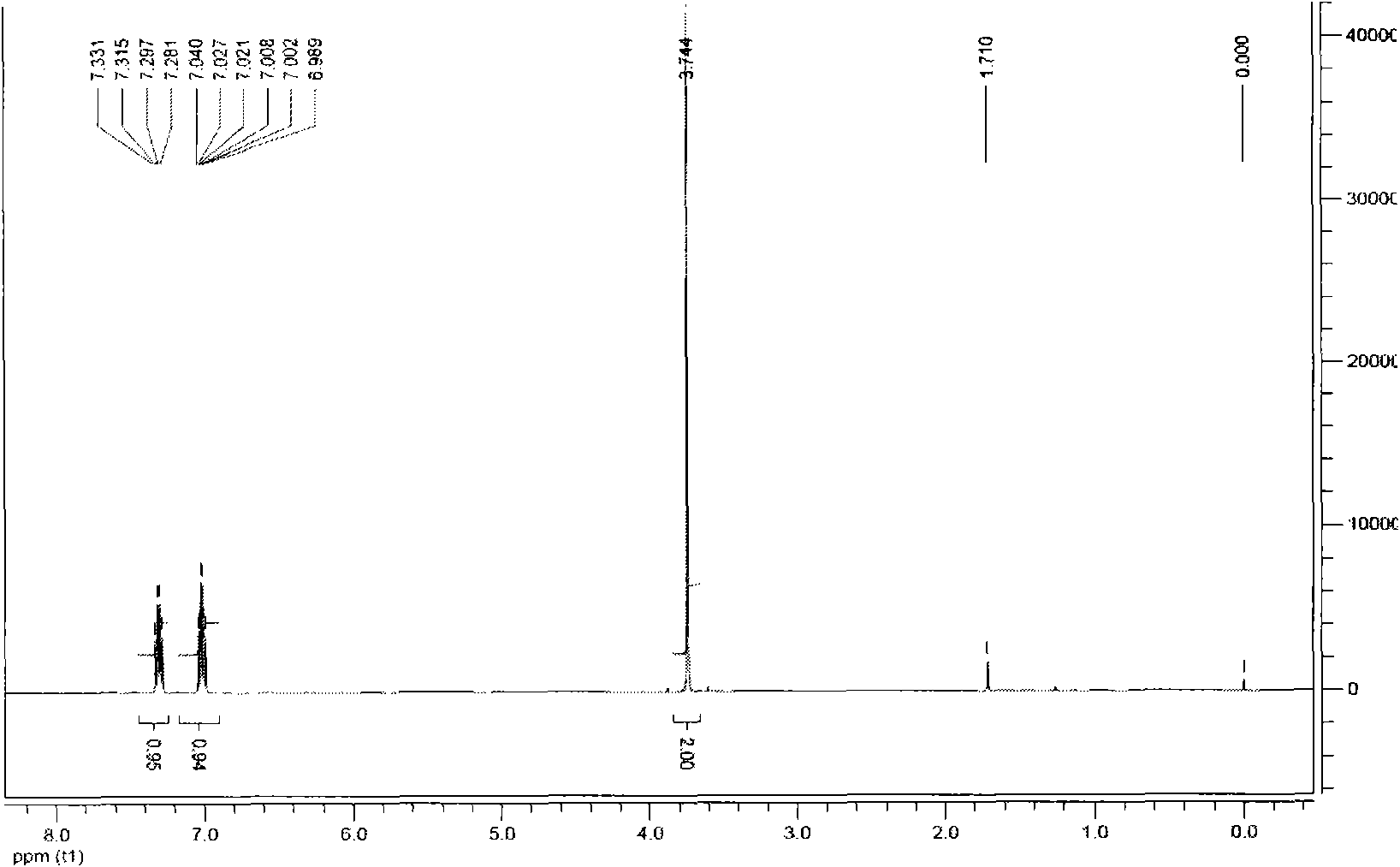
[0038] Add 100ml of 98% sulfuric acid into a 250ml four-necked reaction flask, cool down to 20°C, add 21.3g of paraformaldehyde (equivalent to 0.71mol of monomeric formaldehyde), 44.8g of sodium chloride (0.766mol), and finally add 1,2 , 50.8g (0.385mol) of 4-trifluorobenzene, 40 DEG C heat preservation reaction for 10 hours, the reaction solution was poured into ice water, the organic layer was separated, washed with water until neutral, dried and then distilled under reduced pressure to obtain 2, 58.8g of 4,5-trifluorobenzyl chloride, content 99.8%, yield 84.7%. In the accompanying drawings figure 1 It is the hydrogen spectrum spectrum of 2,4,5-trifluorobenzyl chloride.
[0039] NMR data analysis:
[0040]1H-NMR (CDCl3, 500Hz) δ: 4.56 (s, 2H), 6.95 (m, 1H), 7.26 (m, 1H).
Embodiment 2
[0041] Embodiment 2: the preparation of 2,4,5-trifluorobenzyl chloride
[0042] According to Example 1, 57.1 g (0.766 mol) of potassium chloride was used instead of sodium chloride to obtain 55.1 g of 2,4,5-trifluorobenzyl chloride with a content of 98.2% and a yield of 79.3%.
Embodiment 3
[0043] Embodiment 3: the preparation of 2,4,5-trifluorobenzyl chloride
[0044] According to Example 1, 100 ml of hydrochloric acid was used instead of sulfuric acid to obtain 47.6 g of 2,4,5-trifluorobenzyl chloride with a content of 98.6% and a yield of 68.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com