Preparation method of reactive type water-solubility chitosan derivative
A water-soluble chitosan and reactive technology, which is applied in the field of preparation of reactive water-soluble chitosan derivatives, can solve the problems of increased cost, complicated preparation method, high energy consumption, etc., and can save raw materials and costs, reduce Production cost, the effect of strong controllability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
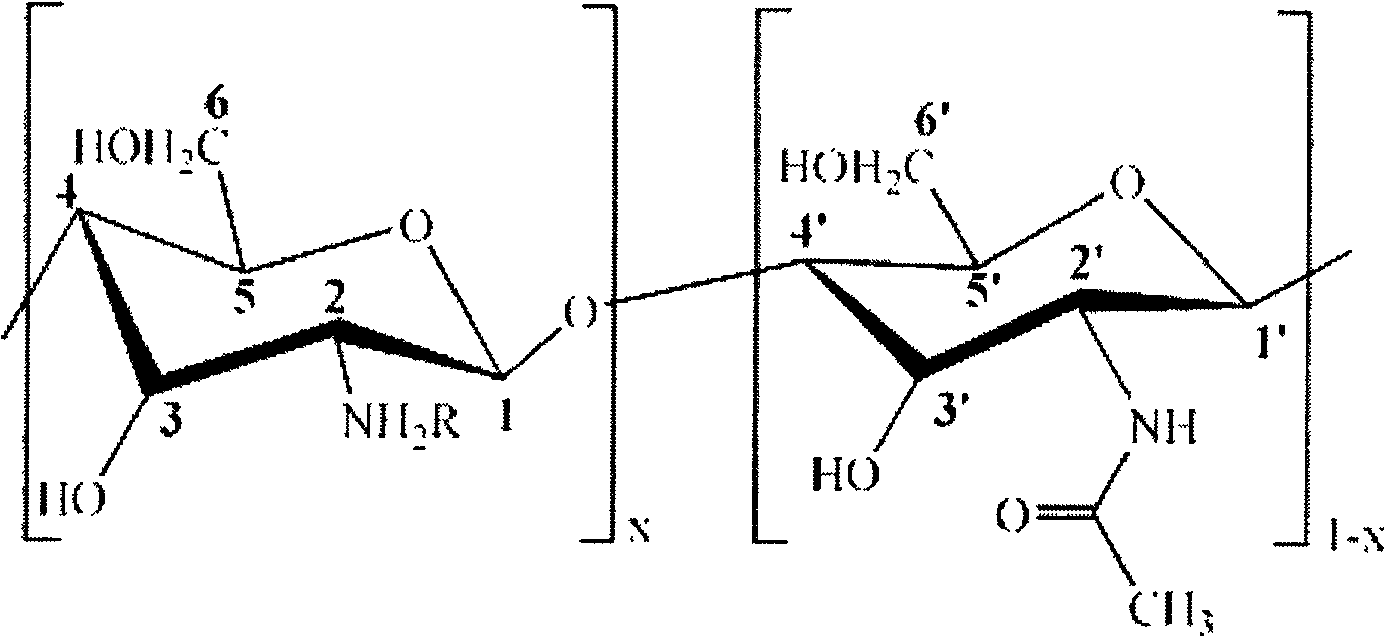
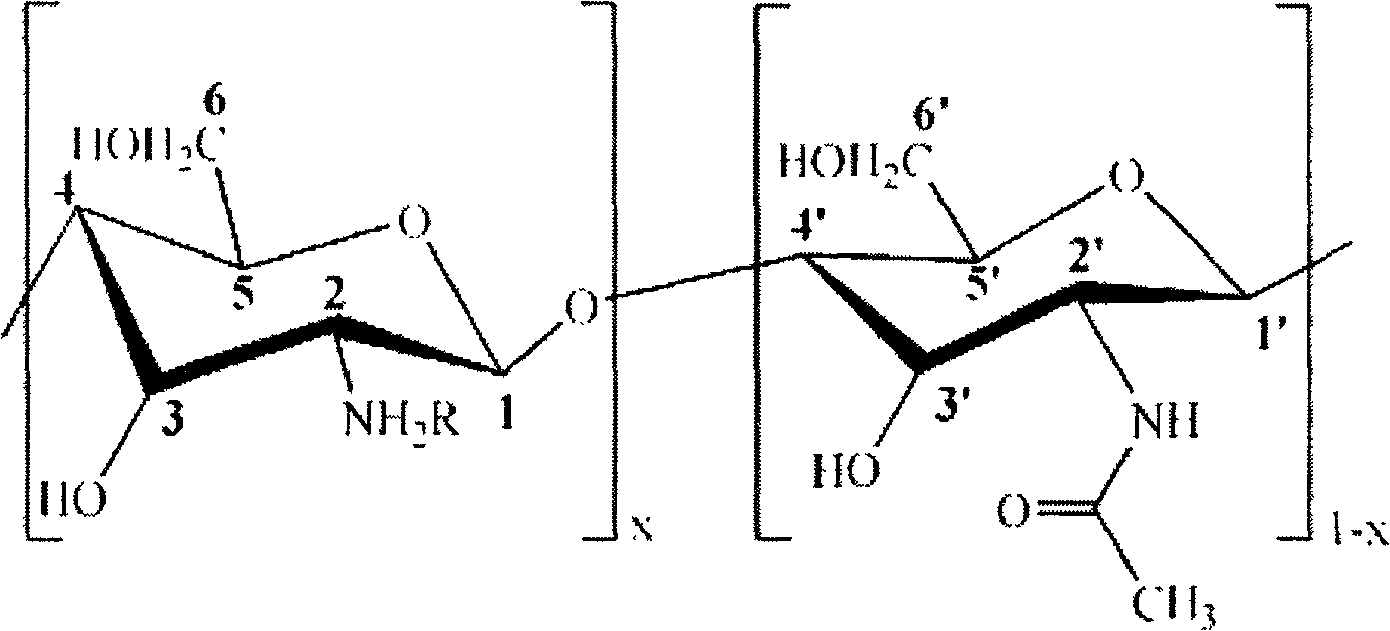
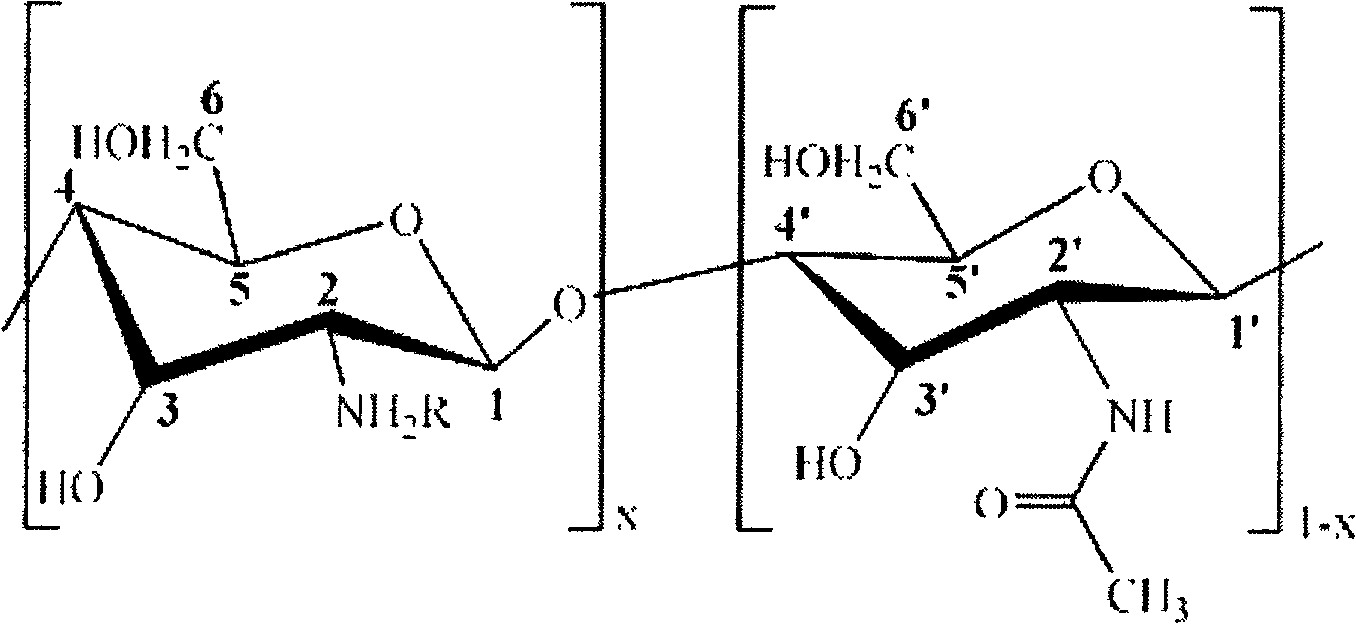
[0031] Add 2.0g chitosan (degree of deacetylation DP=90%, weight average molecular weight Mw=500,000) into 100mL aqueous solution containing 1wt% of 2-acrylamide-2-methylpropanesulfonic acid, stir at room temperature for 0.5 hours, Then add p-hydroxyanisole accounting for 1% of the moles of 2-acrylamide-2-methylpropanesulfonic acid to the above solution, continue stirring at room temperature for 0.5 hours, raise the temperature to 50°C, react for 4 hours, filter and cool, Rotary evaporation to 30mL, then placed in a dialysis bag for dialysis for 48 hours, freeze-dried for 24 hours to obtain a white powdery chitosan derivative with a degree of substitution of 0.6. Its structural formula is as follows.
[0032]
[0033] Among them, R=HO 3 SCH 2 C(CH3) 2 NHCOCH=CH 2 , x is 0.9.
[0034] The aqueous solution of the chitosan derivative with a concentration of 0.1wt% is prepared, which has an absorption peak at ultraviolet 236nm.
Embodiment 2
[0036] 2.0g chitosan (deacetylation degree DP=50%, weight average molecular weight Mw=1,000,000) was added to 100mL aqueous solution containing 2-acrylamide-2-methylpropanesulfonic acid 4wt%, stirred at room temperature for 0.5 hours, Then add 2-acrylamide-2-methylpropanesulfonic acid mole p-hydroxyanisole to the above solution, continue stirring at room temperature for 0.5 hours, raise the temperature to 55°C, react for 3 hours, filter and cool, Rotary evaporation to 30mL, then placed in a dialysis bag for dialysis for 48 hours, freeze-dried for 24 hours to obtain a white powdery chitosan derivative with a degree of substitution of 0.7. Its structural formula is as follows.
[0037]
[0038] Among them, R=HO 3 SCH 2 C(CH3) 2 NHCOCH=CH 2 , x is 0.5.
[0039] Aqueous solution with a concentration of 0.1wt% chitosan derivative is prepared, which has an absorption peak at ultraviolet 240nm.
Embodiment 3
[0041]Add 2.0g chitosan (degree of deacetylation DP=70%, weight average molecular weight Mw=5,000) into 100mL aqueous solution containing 2wt% of 2-acrylamide-2-methylpropanesulfonic acid, stir at room temperature for 0.5 hours, Then add p-hydroxyanisole accounting for 1% of the moles of 2-acrylamide-2-methylpropanesulfonic acid to the above solution, continue stirring at room temperature for 0.5 hours, raise the temperature to 45°C, react for 2 hours, filter and cool, Rotary evaporation to 30mL, then placed in a dialysis bag for dialysis for 48 hours, freeze-dried for 24 hours to obtain a white powdery chitosan derivative with a degree of substitution of 0.3. Its structural formula is as follows.
[0042]
[0043] Among them, R=HO 3 SCH 2 C(CH3) 2 NHCOCH=CH 2 , x is 0.7.
[0044] Aqueous solution with a concentration of 0.1wt% chitosan derivative is prepared, which has absorption peak at ultraviolet 234nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com