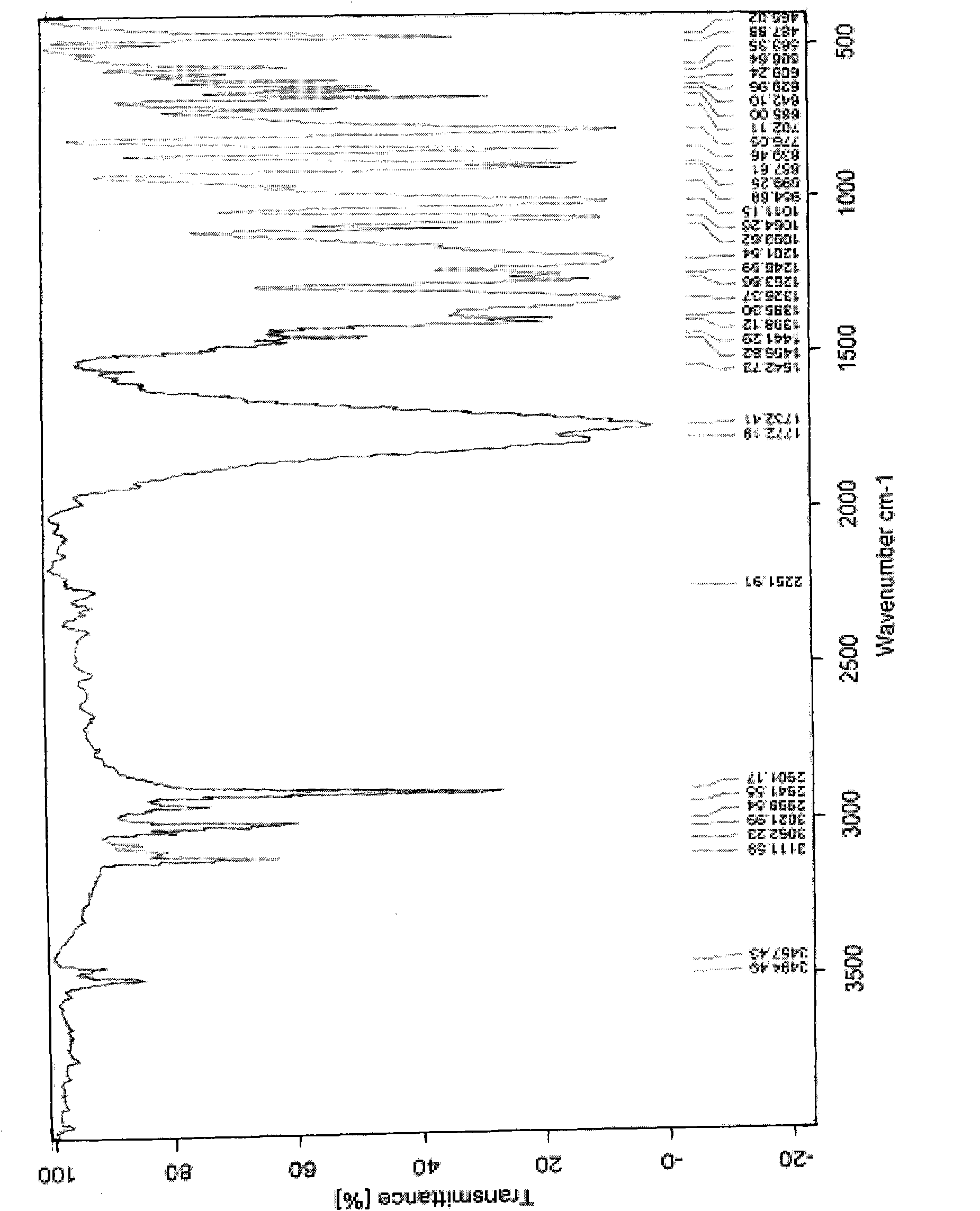
Carboxylic acid penicillin amine salt and application thereof in preparing high-purification sodium oxacillin salt
A technology of penicillin carboxylate and high-purity penicillin carboxylate, which is applied in the application field of preparing high-purity penicillin sodium salt, can solve the problems of lack of reach, high color grade, poor stability, etc., and achieve convenient processing, simple operation and stable properties Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The preparation of embodiment 1 ticarcillin dibenzylethylenediamine salt
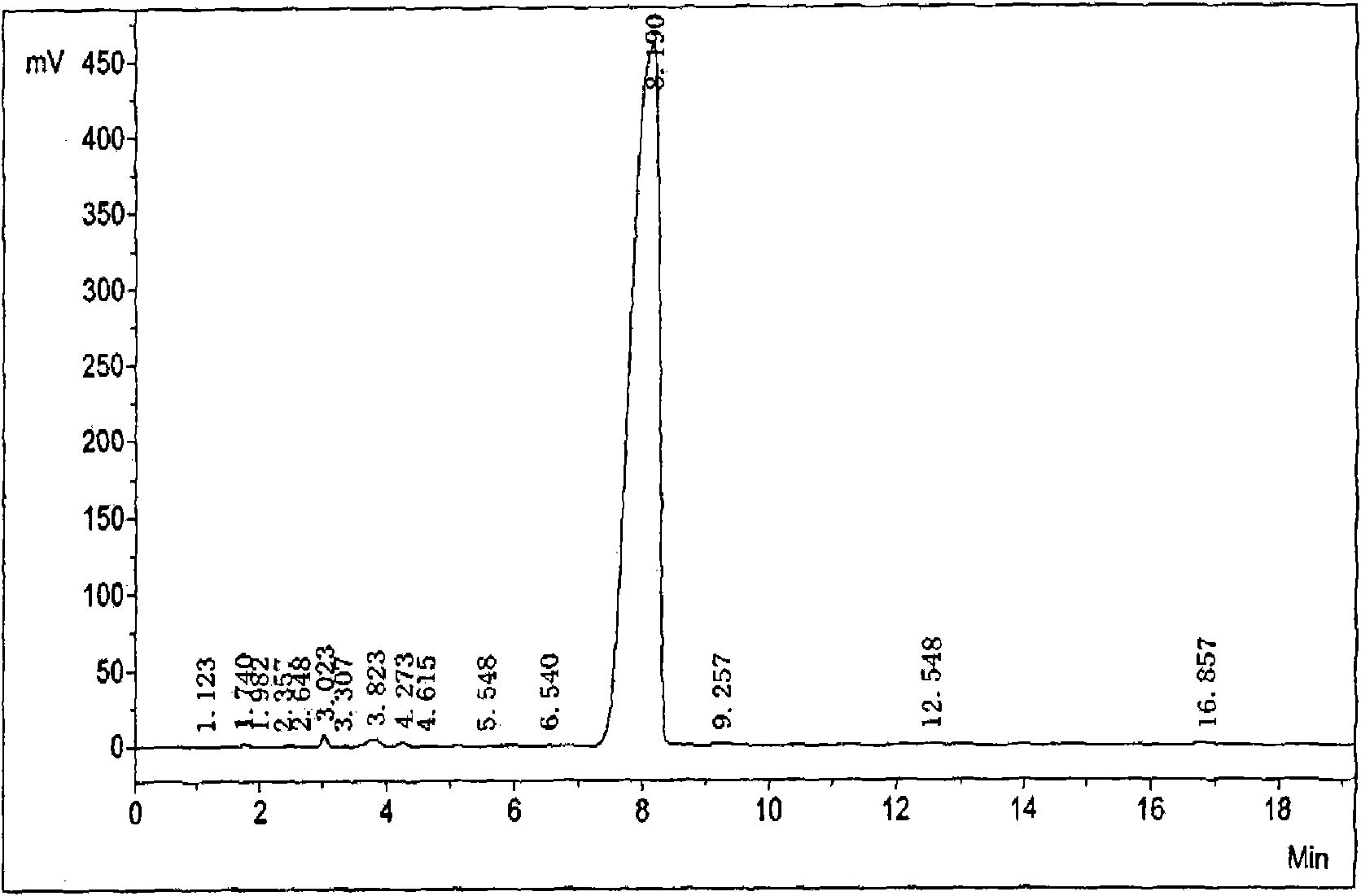
[0065] Dissolve 21 g (0.05 mol) of ticarcillin sodium salt in 190 ml of water, add 200 ml of acetone to dilute, and filter after the solid is completely dissolved. Under rapid stirring of the obtained filtrate, N,N-dibenzylethylenediaminediacetic acid solution (18g (0.05mol) N,N-dibenzylethylenediaminediacetic acid dissolved in 150ml water) was added. A white crystalline solid precipitated out. After stirring for 1 hour, it was filtered and washed with water, and the obtained solid of ticarcillin dibenzylethylenediamine salt was dried at 35°C for 24 hours to obtain a solid weight of 30 g. Yield 86.9%. mp226~230°C (decomposition). High pressure liquid phase (HPLC) detection purity is 99.5% ( figure 2 ).
[0066] 1 HNMR analysis proves that the molar ratio of dibenzylethylenediamine and ticarcillin in the obtained ticarcillin dibenzylethylenediamine salt compound is dibenzylethylenediamine: ...
Embodiment 2
[0068] The preparation of embodiment 2 ticarcillin dibenzylethylenediamine salt
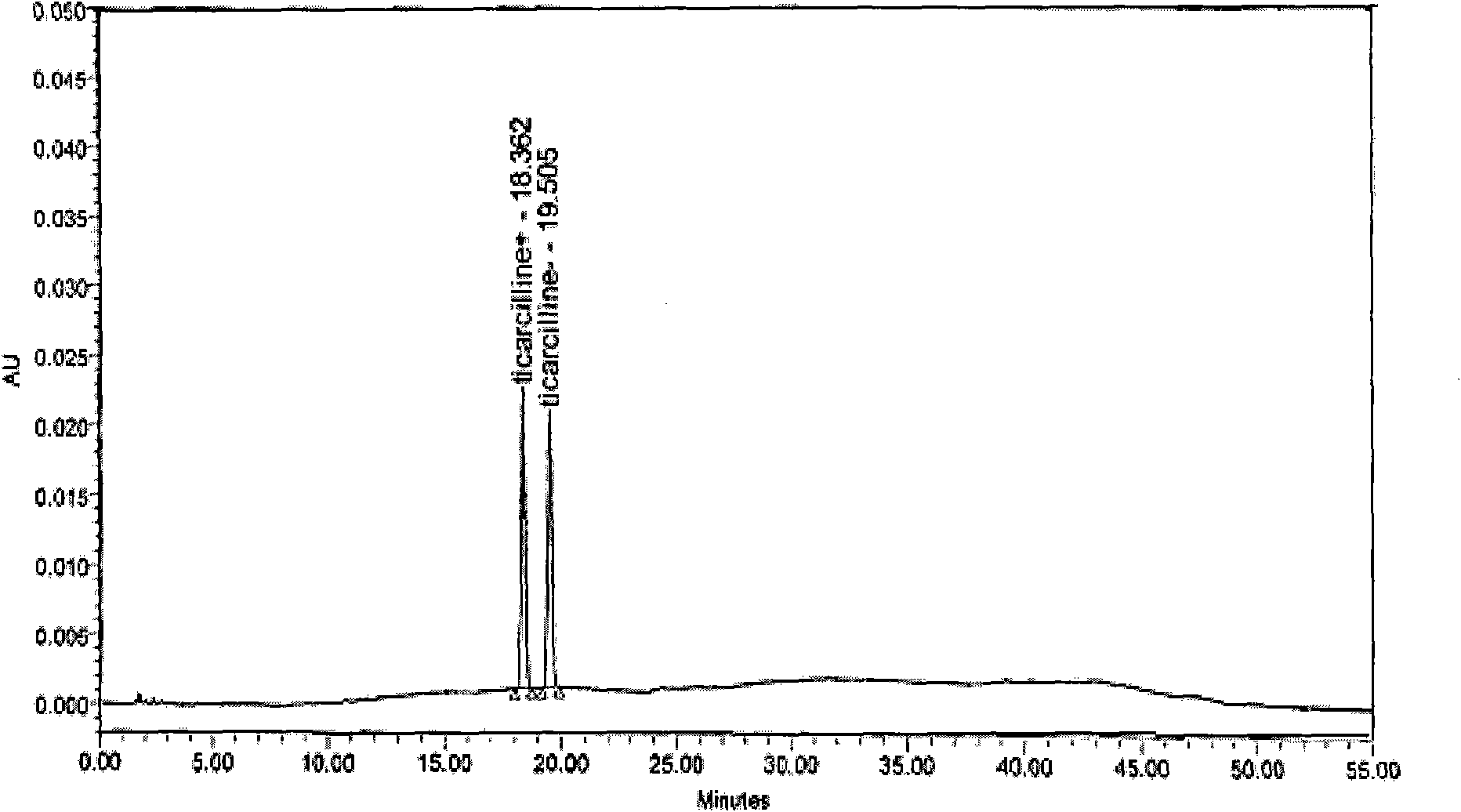
[0069] 4.2 g (0.01 mol) of ticarcillin sodium salt was dissolved in 40 ml of water, and a solution of 3.6 g of N,N-dibenzylethylenediamine diacetic acid and 40 ml of water was added to the feed solution. The ticarcillin benzylethylenediamine salt was precipitated immediately. After stirring for 2 hours, it was filtered, washed with water, and dried under vacuum at 35°C for 24 hours. 6 g of ticarcillin dibenzylethylenediamine salt was obtained with a yield of 79.5%. mp225~228°C, its purity is 99.7% by HPLC. Structure is identical with embodiment 1.
Embodiment 3
[0070] The preparation of embodiment 3 ticarcillin dibenzylethylenediamine salt
[0071] Dissolve 84 g (0.2 mol) of ticarcillin sodium in 420 ml of water, stir at room temperature until dissolved and filter. 61.5 g (0.2 mol) of N, N-dibenzylethylenediamine dihydrochloride was dissolved in 720 ml of water, and added to the ticarcillin sodium solution under rapid stirring. After adding, add 780ml acetone in feed liquid. Stir for 30 minutes, filter, wash with water, and vacuum-dry at 35° C. for 48 hours to obtain 130 g of ticarcillin dibenzylethylenediamine salt. Yield 97%. mp226~228°C, its purity is 99.2% by HPLC. Structure is identical with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com