Method for separating and purifying crude lanosterol product
A technology for lanosterol and crude products, which is applied in the field of separation and purification of lanosterol crude products, can solve the problems of poor crystal purity, low recovery rate, expensive reagents, etc., and achieve high recovery rate, good separation and good purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
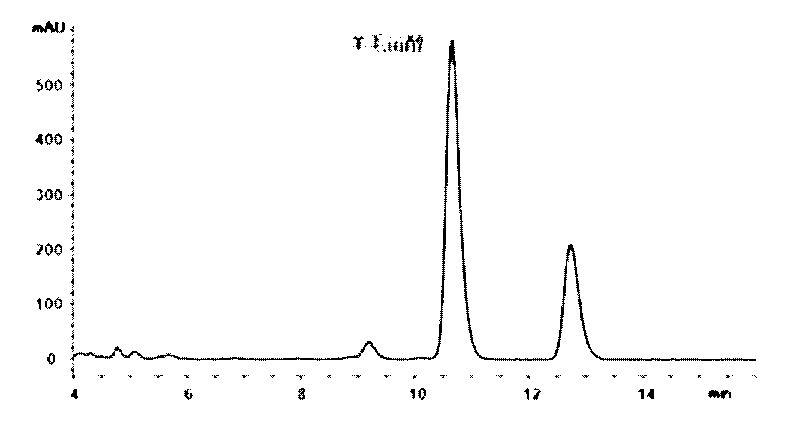
[0024] 1. Weigh more than 1g of crude lanosterol on the market (see HPLC analysis) figure 1 ), add 200mL of methanol, and sonicate for 5 minutes to promote dissolution, place the suspension in a water bath at 25-35°C for 30 minutes, and the supernatant is a saturated methanol solution of crude lanosterol.
[0025] 2. Pass the above saturated solution through a 0.22 μm filter membrane as a preparative chromatographic injection solution. The inner diameter of the preparative chromatographic column is 10 mm, the column length is 250 mm, the column filler is C18 (particle size 5 μm), and the quantitative loop is 2 mL. HPLC grade methanol was used as the elution phase, isocratic elution was performed at a flow rate of 5 mL / min, and the running time was 18 minutes. By on-line UV detector monitoring fraction, detection wavelength is 210nm, according to the peak cutting and collecting effluent, lanosterol 0.65mg can be obtained, the purity is greater than 97%, and the recovery rate i...
Embodiment 2
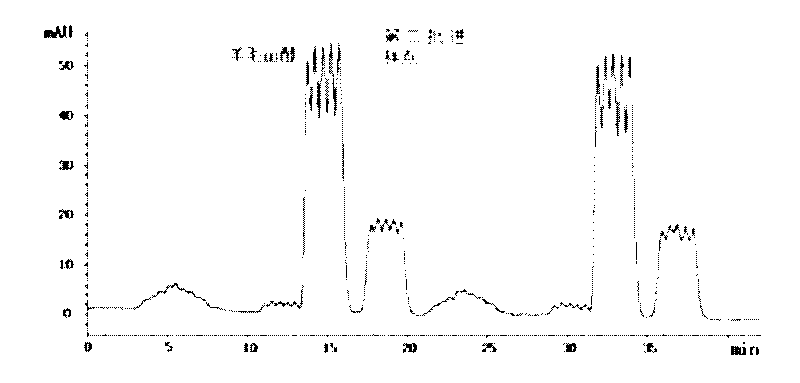
[0030] The preparation method of lanosterol crude methanol saturated solution is the same as that in Example 1, and passed through a 0.22 μm filter membrane as the preparation of chromatographic injection solution. The inner diameter of the preparative chromatographic column is 10mm, the column length is 250mm, the filler is C18 (particle size 5μm), and the quantitative loop is 2mL. Second-rate. HPLC grade methanol was used as the elution phase, isocratic elution was performed at a flow rate of 5 mL / min, and the running time was 22 minutes. Monitor the fraction by an online ultraviolet detector, the detection wavelength is 210nm, cut and collect the effluent according to the peak, and 3.2 mg of lanosterol can be obtained with a purity greater than 97% and a recovery greater than 95%, as figure 2 shown.
Embodiment 3
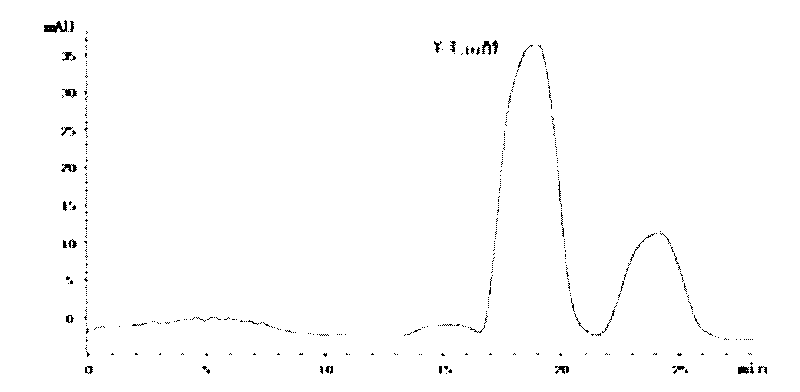
[0032] The preparation method of lanosterol crude methanol saturated solution is the same as that in Example 1, and passed through a 0.22 μm filter membrane as the preparation of chromatographic injection solution. The inner diameter of the preparative chromatographic column is 10mm, the column length is 250mm, the filler is C18 (particle size 5μm), and the quantitative loop is 2mL. Second-rate. HPLC-grade acetonitrile was used as the elution phase, and the flow rate was isocratic at 5 mL / min, and the running time was 22 minutes. Fractions are monitored by an on-line UV detector with a detection wavelength of 210nm, e.g. figure 2 As shown, according to the peak cutting and collecting the effluent, 3.4mg of lanosterol can be obtained, the purity is greater than 97%, and the recovery rate is greater than 95%.
[0033] The above three examples illustrate that the strategy of repeated injection can double the growth efficiency without affecting the purity, which has important a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com