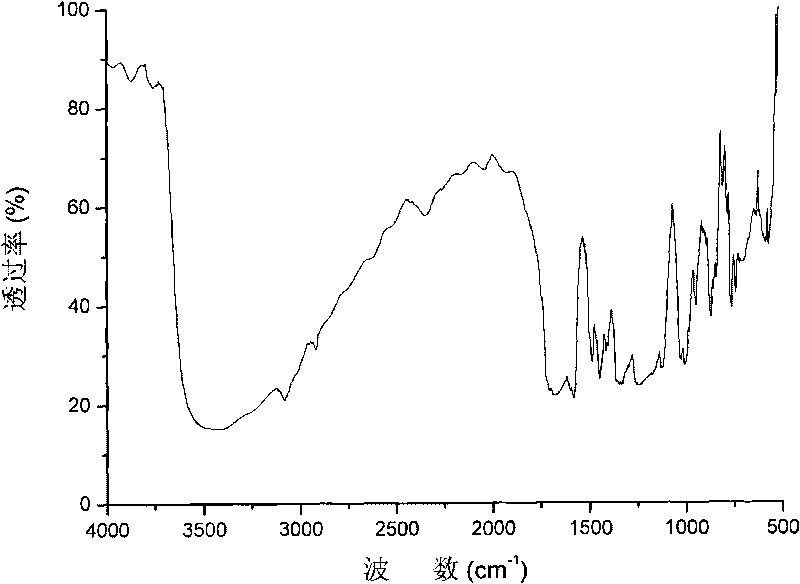
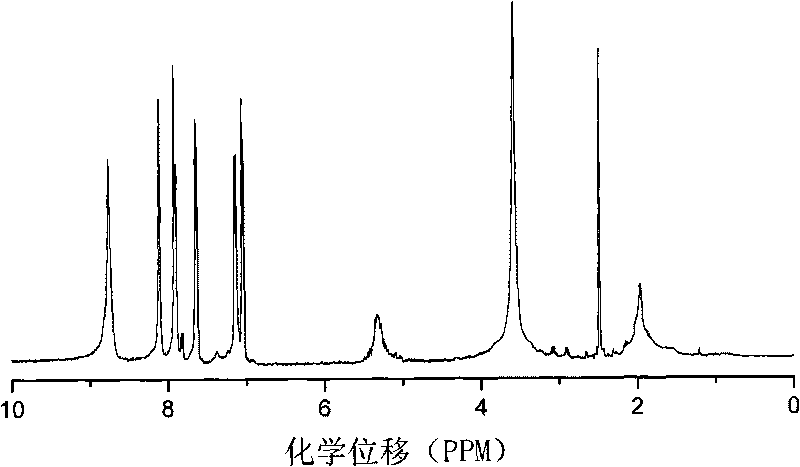
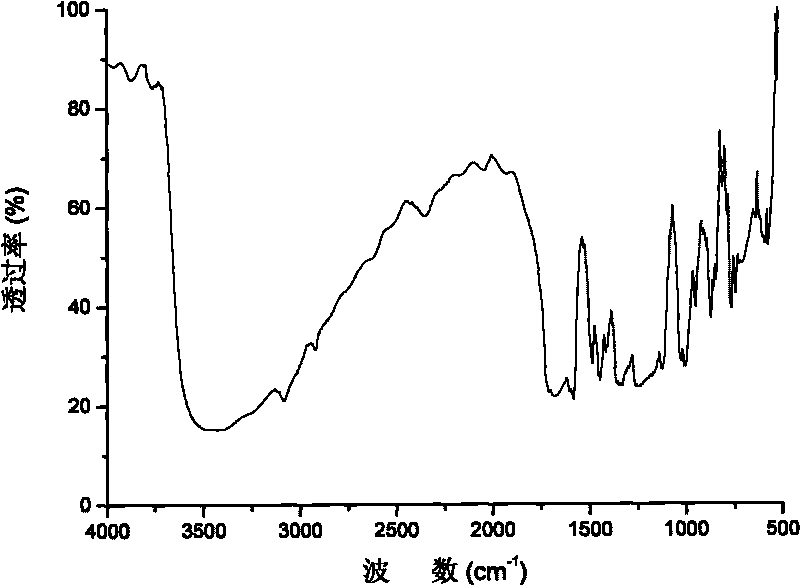
Segmented copolymer of side chain sulfonated type polyimide and sulfonated polybutadiene and preparation method thereof
A technology of sulfonated polybutadiene and block copolymers, which is applied in the direction of electrochemical generators, structural parts, battery pack parts, etc., and can solve problems such as easy loss and high price of perfluorosulfonic acid proton exchange membranes , to simplify the synthesis, ensure the reaction conversion rate and improve the hydrolysis stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (a) Dissolve 3.25g of carboxy-terminated polybutadiene in 30g of toluene, add 3g of thionyl chloride and stir evenly; rise to 60°C for 3 hours under nitrogen protection, then rise to 110°C for 12 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of terminal acid chloride polybutadiene:
[0034] (b) Add 11.525g of 3,5-dinitrobenzoyl chloride and 8.5g of anhydrous aluminum trichloride to a three-neck round bottom flask, and stir slowly under ice bath conditions; slowly add 8.5g of diphenyl diphenyl chloride dropwise within 1 hour After ether, react for 30 minutes; then heat to 50°C under the protection of nitrogen, and continue to react for 12 hours; cool the reaction solution, pour it into ice hydrochloric acid solution to precipitate, stir, filter, wash the crude product, and vacuum dry to obtain 3 , 5-dinitro-4'-phenoxybenzophenone;
[0035](c) Add 3.6...
Embodiment 2
[0044] (a) Dissolve 6.5g of carboxy-terminated polybutadiene in 40g of toluene, add 6g of thionyl chloride and stir evenly; rise to 70°C for 2 hours under nitrogen protection, then rise to 100°C for 15 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of polybutadiene with acyl chloride terminal;
[0045] (b) Add 23.05g 3,5-dinitrobenzoyl chloride and 17g anhydrous aluminum trichloride to the three-neck round bottom flask, and stir slowly under ice bath conditions; after slowly adding 17g diphenyl ether in 1.5 hours , reacted for 60 minutes; then heated to 60°C under the protection of nitrogen, and continued to react for 18 hours; cooled the reaction solution, poured it into ice hydrochloric acid solution to precipitate, stirred, filtered, washed the crude product, and vacuum dried to obtain 3,5 - dinitro-4'-phenoxybenzophenone;
[0046] (c) Add 7.28g 3,5-dini...
Embodiment 3
[0055] (a) Dissolve 9.75g of carboxyl-terminated polybutadiene in 50g of toluene, add 9g of thionyl chloride and stir evenly; rise to 80°C for 1 hour under nitrogen protection, then rise to 90°C for 18 hours After the reaction is finished, cool down, and remove the remaining thionyl chloride by distillation under reduced pressure, so as to obtain the toluene solution of polybutadiene with acyl chloride terminal;
[0056] (b) Add 34.575g 3,5-dinitrobenzoyl chloride and 25.5g anhydrous aluminum trichloride to the three-neck round bottom flask, and stir slowly under ice bath conditions; slowly add 25.5g diphenyl diphenyl chloride dropwise in 2 hours After ether, react for 90 minutes; then heat to 70°C under the protection of nitrogen, and continue to react for 24 hours; cool the reaction solution, pour it into ice hydrochloric acid solution for precipitation, stir, filter, wash the crude product with water, and vacuum dry to obtain 3 , 5-dinitro-4'-phenoxybenzophenone;
[0057] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com