Magnetic medicament loading method for immunosuppressant
An immunosuppressant and magnetic technology, applied in the fields of polymer materials and biomedicine, can solve the problems of limited application, low encapsulation rate, non-magnetic properties, etc., and achieve the effects of being favorable for popularization, low cost and large drug loading capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A mixture of cyclohexanone and cyclohexane at a volume ratio of 3:1 was added to tacrolimus to prepare a 1 mg / ml immunosuppressant solution. Take 5 mg of magnetic particles and add them into a 10 ml pear-shaped bottle, then add 1 mg of the prepared tacrolimus solution, shake for 48 hours at 25 ° C on a shaking table with a rotation speed of 200 rpm, and reach the balance of drug loading, and then use a rotary evaporator to dissolve the organic The solvent was evaporated and stored at 4°C to obtain magnetic composite particles loaded with tacrolimus. The remaining tacrolimus in the solution was measured by high-performance liquid chromatography, and the following two formulas were used to calculate the drug loading amount to be 20%, and the encapsulation efficiency to be 90%.
[0021]
[0022]
Embodiment 2
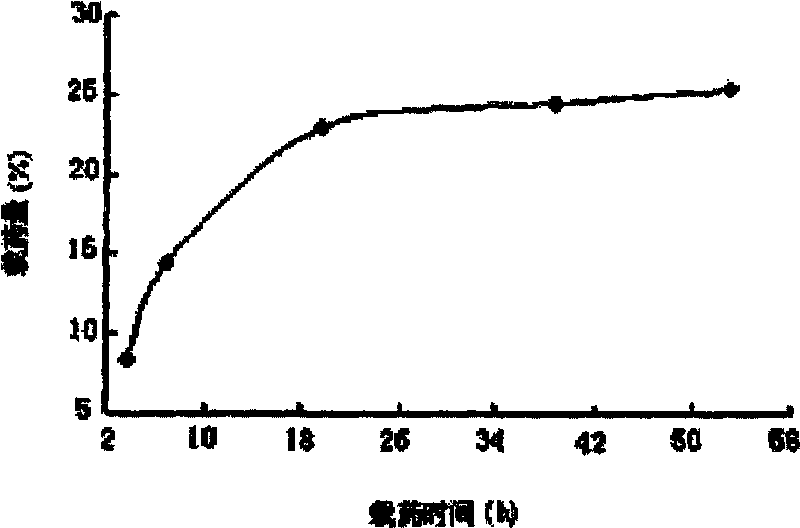
[0024] Prepare a 3mg / ml rapamycin solution with methanol. Add 8mg of magnetic particles into a 10ml pear-shaped bottle, add 2.4mg of rapamycin solution, shake at 25°C for 54h on a shaker with a rotation speed of 180rpm, to reach drug loading balance, and use a rotary evaporator to evaporate the organic solvent and stored at 4°C, the magnetic composite microparticles loaded with rapamycin can be obtained. Measure the remaining rapamycin in the solution by high performance liquid chromatography, and then use the following two formulas to calculate the drug loading, encapsulation efficiency, and the resulting drug loading is 25%, see figure 1 , The encapsulation rate is 95%.
[0025]
[0026]
Embodiment 3
[0028] A 5 mg / ml mycophenolate mofetil solution was prepared with tetrahydrofuran and cyclohexane at a volume ratio of 2:3. Take 10 mg of magnetic particles and add them into a 10 ml pear-shaped bottle, add 4 mg of mycophenolate mofetil solution, and shake for 63 h on a shaking table with a rotation speed of 190 rpm at 25 ° C to achieve drug loading balance. Use a rotary evaporator to evaporate the organic solvent. Stored at 4°C, the magnetic composite particles loaded with mycophenolate mofetil can be obtained. The remaining mycophenolate mofetil in the solution was measured by high performance liquid chromatography, and then the drug loading and encapsulation efficiency were calculated by using the following two formulas. The obtained drug loading was 30%, and the encapsulation efficiency was 92%.
[0029]
[0030]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com