Aminophylline slow-release capsules and preparation method thereof
A technology of sustained-release capsules and aminophylline, which is applied in the field of medicine, can solve the problems of poor oral absorption and low bioavailability, and achieve the effects of improving poor taste, good reproducibility, and good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
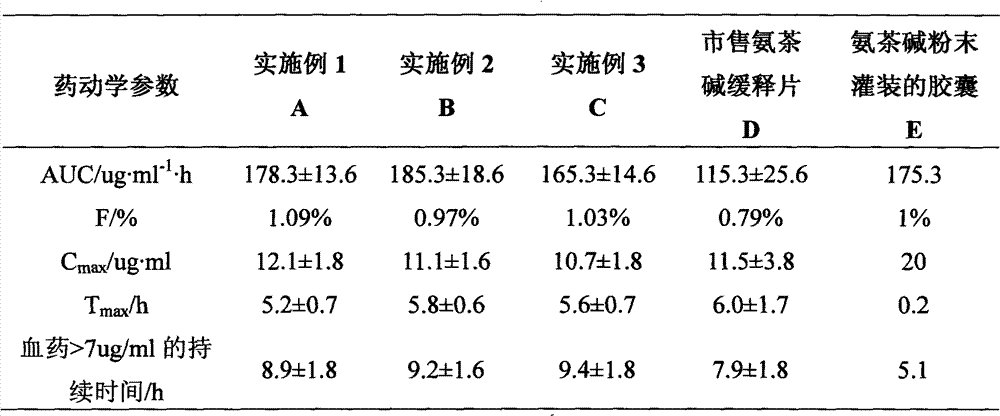
Embodiment 1
[0031] A kind of aminophylline sustained-release capsule comprises the following components by weight percentage:
[0032] Aminophylline 50%,
[0033] Sodium Carboxymethyl Cellulose 18%,
[0034] Magnesium Stearate 0.5%,
[0035] Ethyl Cellulose 20%,
[0036] Polyethylene glycol (PEG1540) 11.5%.
[0037] The production method is as follows:
[0038] (1) Weigh aminophylline and sodium carboxymethylcellulose, add water with a total weight of 10% (w / w), mix them evenly to make a soft material, granulate with a 20-mesh sieve, and dry at 60°C;
[0039] (2) After adding magnesium stearate and mixing, press into a medicine chip with a diameter of 2mm;
[0040] (3) Take ethyl cellulose and polyethylene glycol, add its gross weight 5% (w / w) acetone to dissolve as coating solution;
[0041] (4) Carry out fluidized bed coating to the aminophylline drug chip obtained in step (2) using the coating solution obtained in step (3), the air inlet temperature is 50°C, the air inlet volume ...
Embodiment 2
[0044] A kind of aminophylline sustained-release capsule comprises the following components by weight percentage:
[0045] Aminophylline 48%,
[0046] Hydroxypropyl Methyl Cellulose 17%,
[0047] Talc 0.5%,
[0048] Eudragit RS 100 25%,
[0049] Polysorbate 20 9.5%.
[0050] The production method is as follows:
[0051] (1) Weigh aminophylline and hydroxypropyl methylcellulose, add water with a total weight of 10% (w / w), mix them evenly to make a soft material, granulate with a 20-mesh sieve, and dry at 60°C;
[0052] (2) After adding talcum powder and mixing evenly, press it into a medicine chip with a diameter of 2mm;
[0053] (3) Weigh Eudragit RS 100 and polysorbate 20, add 5% (w / w) of its total weight in isopropanol to dissolve as coating solution;
[0054] (4) Carry out fluidized bed coating to the aminophylline drug chip obtained in step (2) using the coating solution obtained in step (3), the air inlet temperature is 50°C, the air inlet volume is 2g / min, and the ...
Embodiment 3
[0057] A kind of aminophylline sustained-release capsule comprises the following components by weight percentage:
[0058] Aminophylline 55%,
[0059] Carbopol 15%,
[0060] Micronized silica gel 0.8%,
[0061] Eudragit RL100 21.2%,
[0062] Polysorbate 20 8%.
[0063] The production method is as follows:
[0064] (1) Weigh aminophylline and carbopol, add 10% (w / w) water of its total weight and mix evenly to make a soft material, granulate with a 20-mesh sieve, and dry at 60°C;
[0065] (2) Add micropowder silica gel and mix well, then press it into a 2mm diameter drug chip;
[0066] (3) Weighing Eudragit RL100 and polysorbate 20, adding 5% (w / w) of its total weight in isopropanol to dissolve as a coating solution;
[0067] (4) Carry out fluidized bed coating to the aminophylline drug chip obtained in step (2) using the coating solution obtained in step (3), the air inlet temperature is 45°C, the air inlet volume is 2.5g / min, and the liquid spray speed is 2.0g / min , to pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com