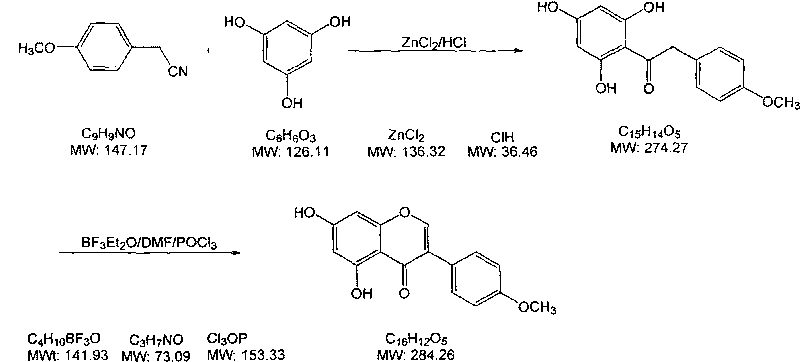
Method for synthesizing biochanin A
A biochanin and a synthesis method technology, applied in the field of synthesis of biochanin A, can solve the problems of high cost, low solubility, harsh reaction conditions, etc., and achieve the effects of reduced production cost, increased solubility, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
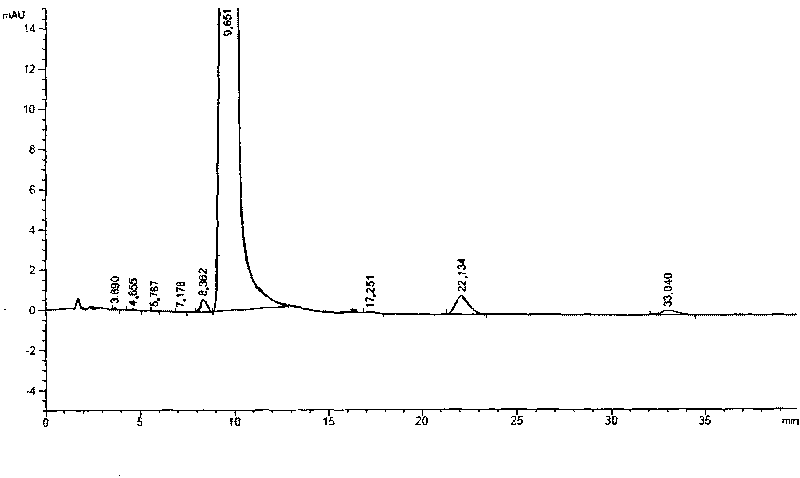
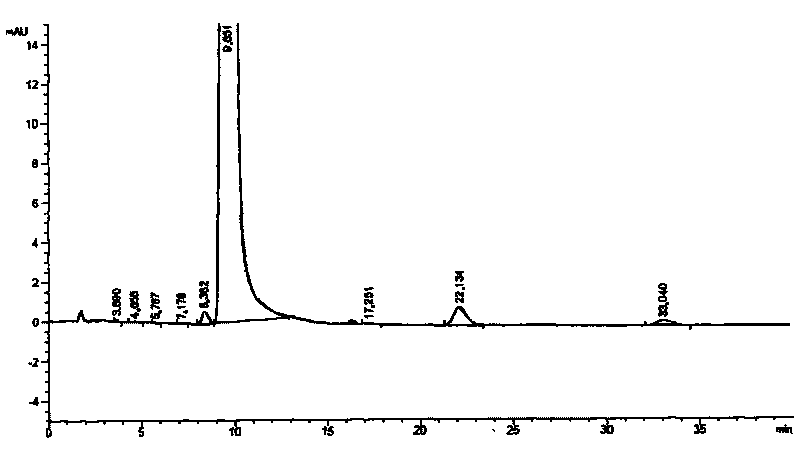
Image
Examples
Embodiment 1
[0023] 1) 20g of p-methoxyphenylacetonitrile and 20g of phloroglucinol were added to a 1000mL four-neck flask, then 10g of anhydrous zinc chloride and 400g of isopropyl ether were added, while hydrogen chloride gas was introduced into the mixture to saturation, and The reaction was stirred for 3 hours under ice-bath conditions;
[0024] 2) The temperature of the above-mentioned reacted mixture was raised to room temperature, and the stirring reaction was continued for 16 hours, then the stirring was stopped, left to stand, and the upper organic layer was poured out, then 500 g of 2% hydrochloric acid solution was added to the residue, and heated to reflux for 3 hours , cooled and filtered to obtain 28.1g intermediate, the yield is 75.4%;
[0025] 3) Mix 25.0 g of the above-mentioned intermediate with 50 g of boron trifluoride ether solution in a container, and then add 150 g of N,N-dimethylformamide dropwise at a temperature of 10° C. to obtain solution A;
[0026] 4) At a te...
Embodiment 2
[0030] 1) 20g p-methoxyphenylacetonitrile and 20g phloroglucinol were added to a 1000mL four-neck flask, then 20g anhydrous zinc chloride and 400g isopropyl ether were added, while hydrogen chloride gas was introduced into the mixture to saturation, and The reaction was stirred for 3 hours under ice-bath conditions;
[0031] 2) The temperature of the above-mentioned reacted mixture was raised to room temperature, and the stirring reaction was continued for 16 hours, then the stirring was stopped, and the upper organic layer was poured out, then 500 g of 2% hydrochloric acid solution was added to the residue, and heated to reflux for 3 hours , cooled and filtered to obtain 35.6g intermediate, the yield is 95.5%;
[0032] 3) Mix 30.0 g of the above-mentioned intermediate with 60 g of boron trifluoride ether solution in a container, and then add 240 g of N,N-dimethylformamide dropwise at a temperature of 10° C. to obtain solution A;
[0033] 4) At a temperature of 10°C, add 60g ...
Embodiment 3
[0037] 1) 20g p-methoxyphenylacetonitrile and 20g phloroglucinol were added to a 1000mL four-necked flask, then 20g anhydrous zinc chloride and 400g n-butyl ether were added, while hydrogen chloride gas was passed into the mixture to saturation, and The reaction was stirred for 3 hours under ice-bath conditions;
[0038] 2) The temperature of the above-mentioned reacted mixture was raised to room temperature, and the stirring reaction was continued for 16 hours, then the stirring was stopped, left to stand, and the upper organic layer was poured out, then 500 g of 2% hydrochloric acid solution was added to the residue, and heated to reflux for 3 hours , cooling, filtering, to obtain 32.4g intermediate, the yield is 86.9%;
[0039] 3) Mix 30.0 g of the above-mentioned intermediate with 60 g of boron trifluoride ether solution in a container, and then add 300 g of N,N-dimethylformamide dropwise at a temperature of 10° C. to obtain solution A;
[0040] 4) At a temperature of 10°...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com