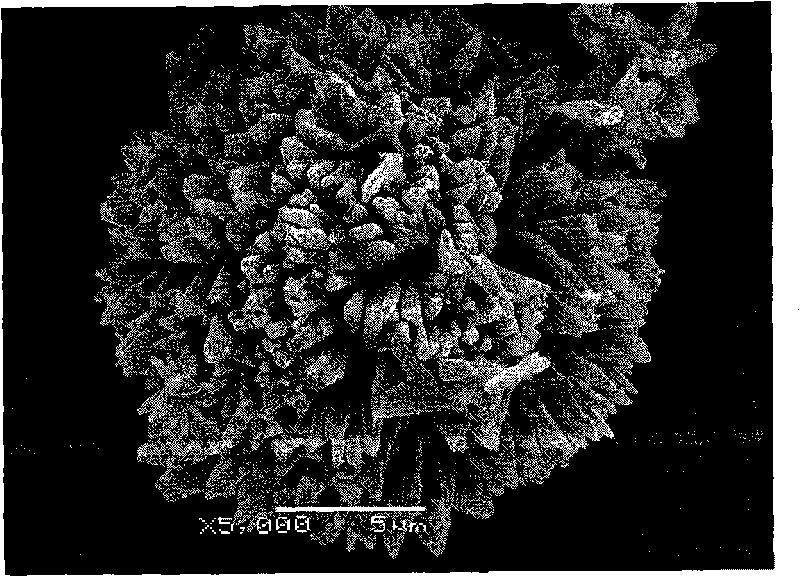
Method and device for preparing medicament particles with nano-micro structure
A technology for preparing a device and a drug, which is applied in the field of preparing nano-micro structure drug particles, can solve the problems of inability to meet nano-micro structure requirements, difficult to control particle size, incomplete product crystallization, etc. control, the effect of narrow particle size distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
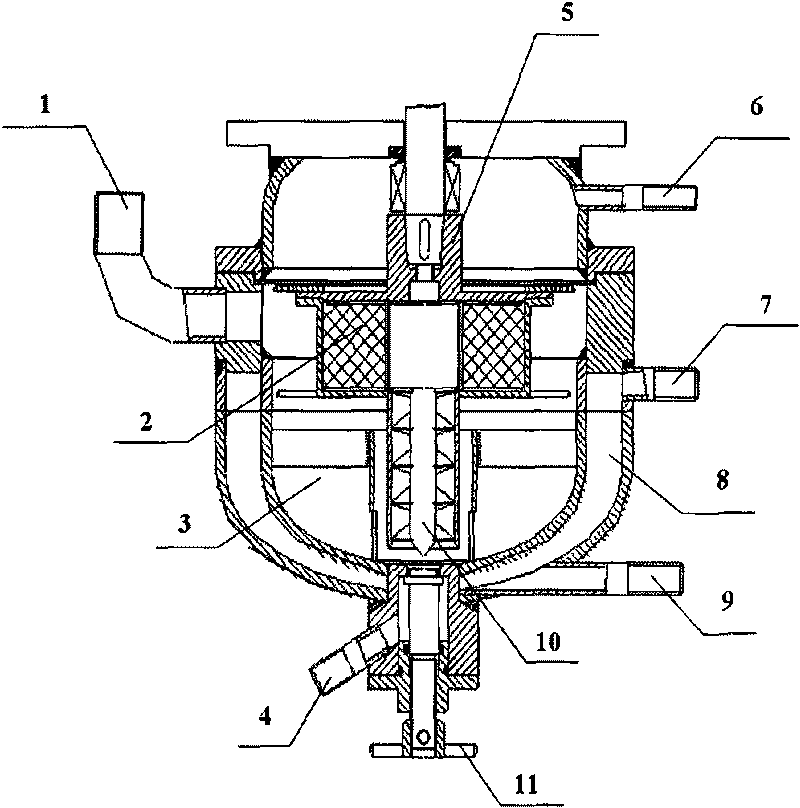
[0038] The reaction apparatus used is as figure 1 As shown, the volume of the cavity 3 is 1200mL, and the filler 2 is an ordinary wire mesh.
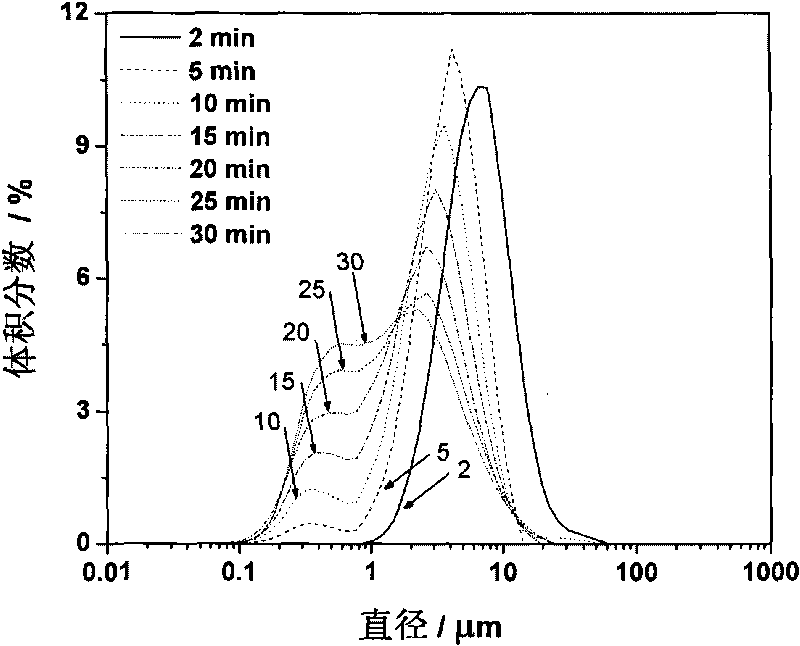
[0039] Accurately weigh 10.0 ± 0.1g salbutamol raw material drug, dissolve it in isopropanol (IPA) at 25°C, and prepare a 0.04mol / L salbutamol IPA solution with a volume constant to 1000mL, and add it into the internal circulation RPB through the feed port 1 Cavity 3 (hereinafter referred to as cavity 3). Water at 25° C. is passed continuously into the RPB jacket 8 . Turn on the digital frequency regulator that controls the motor to 50Hz. The albuterol IPA solution continuously circulates in the cavity 3 under the joint action of the lifter 10 and the rotor 5 . The porosity of the packing layer 2 is 90%. When the number displayed on the frequency controller rises from 0 to 50 Hz and displays stably for 10 seconds, 10 mL of 2.0 mol / L sulfuric acid is quickly added to the internal circulation RPB through the feed port 1, fully mixed w...
Embodiment 2
[0042] The internal circulation RPB used is the same as that in Example 1.
[0043] Accurately weigh 3.0±0.1g (0.01mol) of sumatriptan raw material and dissolve it in tetrahydrofuran (THF), and use the prepared sumatriptan THF solution to 500mL, and add it into the internal circulation RPB through feed port 1 . Turn on the digital frequency regulator controlling the motor to 50 Hz, and the porosity of the packing layer 2 is 90%. Accurately weigh 2.4±0.1g (0.02mol) of succinic acid (also called succinic acid) and dissolve it in tetrahydrofuran (THF), and dilute to 200mL to prepare a succinic acid THF solution. When the number displayed on the FM instrument rises from 0 to 50Hz and displays stably for 10 seconds, quickly add the above-mentioned succinic acid THF solution into the internal circulation RPB through the feed port 1, and fully mix with the sumatriptan THF solution , the reaction crystallized to generate the desired sumatriptan succinate. The mixing time was 6 min,...
Embodiment 3
[0045] The internal circulation RPB used is the same as that in Example 1.
[0046] Accurately weigh 3.0±0.1g (0.01mol) of sumatriptan raw material and dissolve it in tetrahydrofuran (THF), and use the prepared sumatriptan THF solution to 500mL, and add it into the internal circulation RPB through feed port 1 . Turn on the digital frequency regulator controlling the motor to 50 Hz, and the porosity of the packing layer 2 is 90%. Accurately weigh 2.4±0.1g (0.02mol) of succinic acid (also called succinic acid) and dissolve it in tetrahydrofuran (THF), and dilute to 200mL to prepare a succinic acid THF solution. When the number displayed on the FM instrument rises from 0 to 50Hz and displays stably for 10 seconds, quickly add the above-mentioned succinic acid THF solution into the internal circulation RPB through the feed port 1, and fully mix with the sumatriptan THF solution , the reaction crystallized to generate the desired sumatriptan succinate. The mixing time was 6 min,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com