Oligomerization pineapple ginseng glycosaminoglycan and preparation method thereof
一种参糖胺、聚糖的技术,应用在低聚凤梨参糖胺聚糖及其制备领域,能够解决侧链岩藻糖基及硫酸化程度差异、FGAG应用价值差异等问题,达到治疗血栓性疾病的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0080] Example 1 THG Extraction, Depolymerization and Refining
[0081] 1.1 Materials
[0082] Pineapple ginseng, also known as plum flower ginseng (Thelenota ananas), is commercially available, and the internal organs are removed to dry the body wall.
[0083] Sea cucumber (Stichopus japonicus), commercially available, eviscerated and dried body wall.
[0084] Yuzu sea cucumber (H.leucospilota), commercially available, eviscerated and dried body wall.
[0085] Papain: 8 x 10 5 U / g, Nanning Pangbo Bioengineering Co., Ltd., Guangxi.
[0086] Cation exchange resin: 001×7 strongly acidic styrene-based cation exchange resin, Nankai University Resin Factory, Tianjin.
[0087] KOH, KCOCH 3 、H 2 o 2 , ethanol and other reagents are commercially available analytical reagents.
[0088] 1.2 Extraction
[0089] Take 20kg of dried pineapple ginseng, slice it into thin slices with a thickness of about 1.5mm, put it into a sandwich reaction tank (300L), add 200L of water, stir an...
Embodiment 2
[0112] Embodiment 2 THG / dTHG spectral analysis
[0113] Test samples: THG, dTHG-6, dTHG-9, the sources are the same as in Example 1.
[0114] Reference samples: SJG, SJG-1, the source is the same as in Example 1.
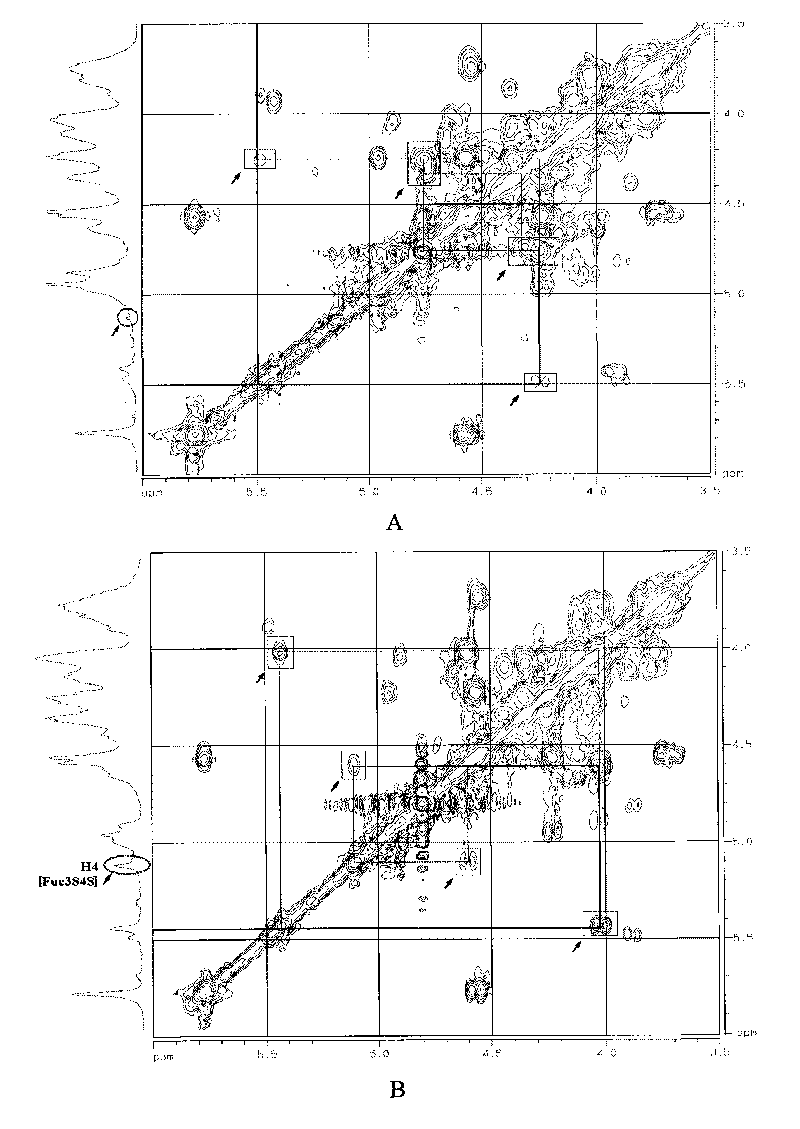
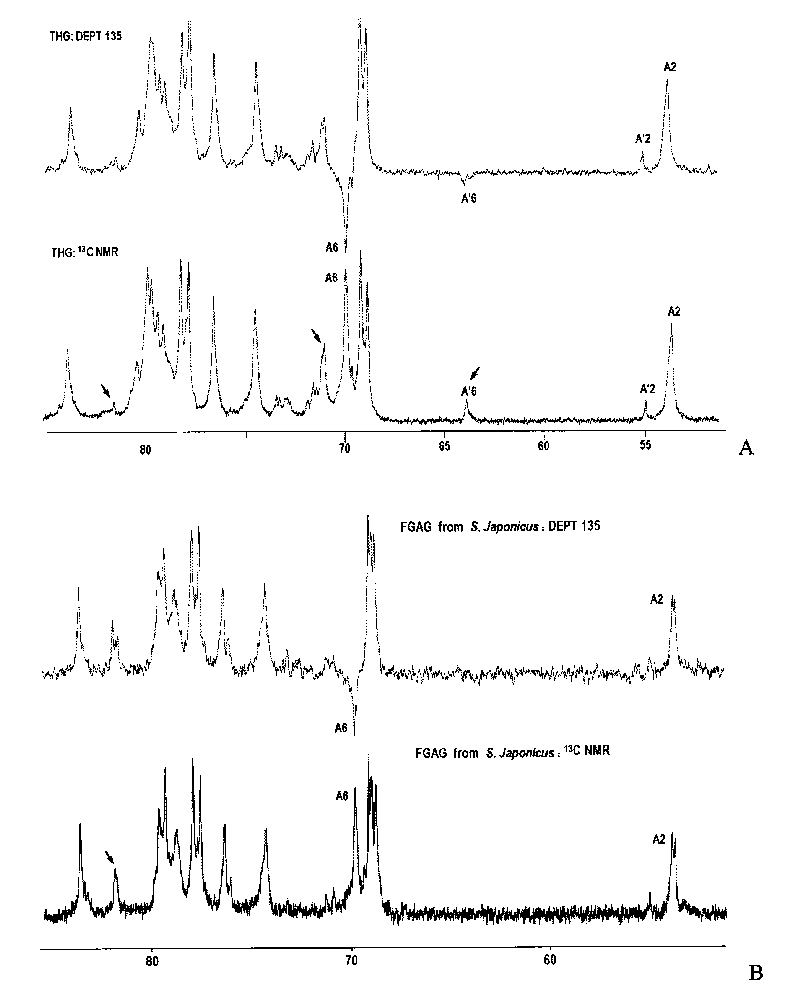
[0115] Detection spectrum: 1 H NMR; 1 H- 1 H COZY; 1 H- 1 H TOCSY; 1 H- 1 H NOESY; 13 C-NMR; DEPT-135°; 1 H- 13 C HSQC; 1 H- 13 C HMBC.
[0116] Detection conditions: solvent, D 2 O, 99.9 Atom% D (Norell); internal standard, trimethylsilyl-propionic acid (TSP-d4); temperature, 45°C.
[0117] Instrument, AVANCEAV 400 superconducting nuclear magnetic resonance spectrometer (Bruker, Switzerland, 400MHz).
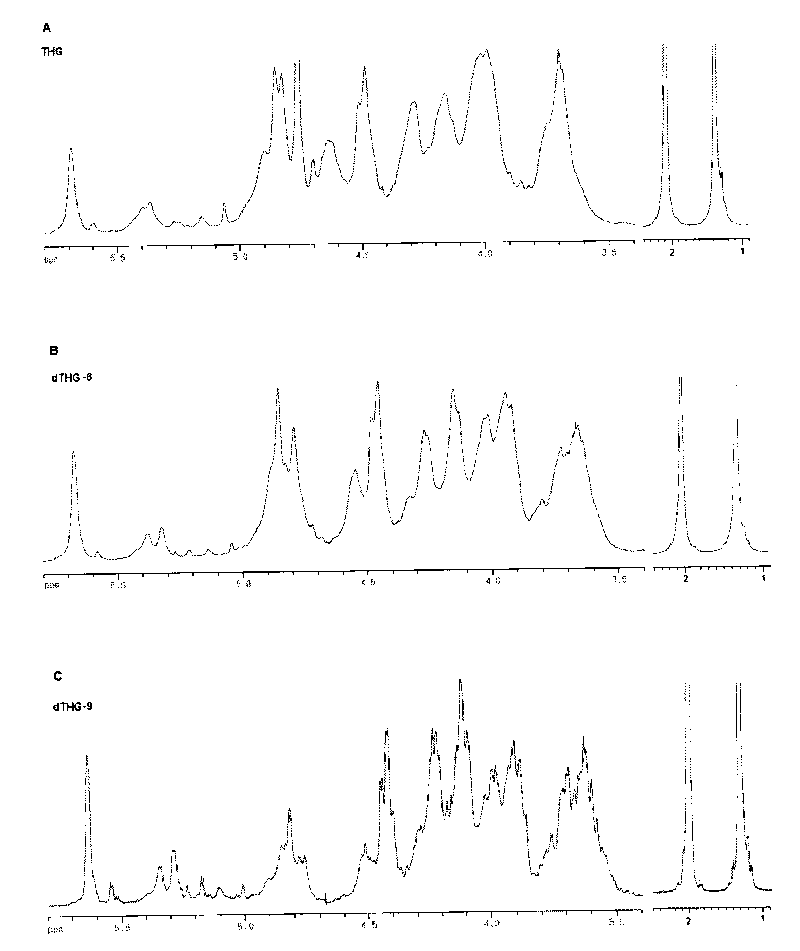
[0118] Spectrum: see Figure 1 ~ Figure 4 .
[0119] Result analysis:
[0120] (1) Spectrum comparison between THG and dTHG:
[0121] figure 1 Display of THG, dTHG-6, dTHG-9 1 H NMR spectrum, in which the water peaks in dTHG-6 and dTHG-9 spectra are suppressed. From figure 1 It can be seen that the signal characteristics of THG, dTHG-6 and dT...
Embodiment 3
[0133] Example 3 THG / dTHG Biological Activity Detection
[0134] 3.1 Detection of platelet-inducing activity
[0135] Sample: dTHG-1~dTHG-10, source is the same as Example 1
[0136] Reference samples: dSJG-1~dSJG-4, the source is the same as in Example 1
[0137] Methods: New Zealand white rabbits were taken from the abdominal aorta, anticoagulated with 3.8% sodium citrate (1:9), centrifuged at 200×g for 8 minutes to obtain platelet-rich plasma (PRP), and centrifuged at 1500×g for 10 minutes to obtain platelet-poor plasma. PRP platelet count about 4.0×10 5 / mm 3 . Bron method (Born GVR. Nature, 1962, 194: 927) platelet aggregometer to detect the effect of samples on platelet aggregation. In the experiment, normal saline was used as the blank control, the final concentration of the sample was 200 μg / ml, the experiment was repeated three times, and the mean value of the maximum aggregation rate of platelets was calculated.
[0138] Results: See Table 4.
[0139] Table ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com