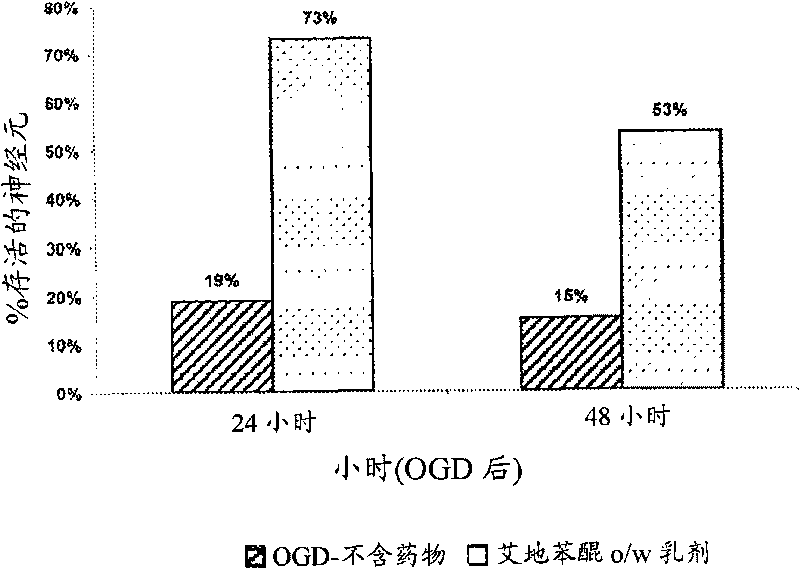
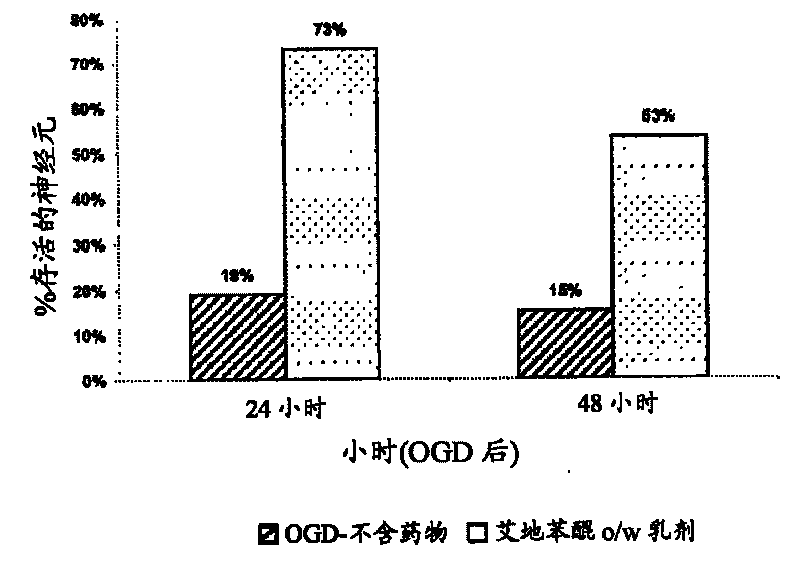
Idebenone composition for the treatment of neurological disorders
A technique for neurological disorders and compositions, applied in the field of stable preparations of idebenone, capable of solving problems such as low solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-10
[0031] Examples 1-10: Oil-in-Water Emulsions of Idebenone
Embodiment 1
[0032] Example 1. Preparation of injectable idebenone o / w (oil-in-water) emulsion
[0033] The oil components of the formulation (Capric / caprylic triglyceride, acetylated monoglyceride and D-alpha-tocopherol USP) were combined with lecithin and ethoxylated castor oil and mixed for 1 hour at 40°C. Idebenone was dissolved in a warm oil and surfactant mixture and then mixed with water, Aqueous blend of EDTA and glycerin. The resulting emulsion is subjected to a high-pressure homogenizer (eg Avestin TM Emulsiflex C5) was treated 3-5 times. After cooling to room temperature, the emulsion was aseptically filtered through a sterile microporous membrane filter (0.2 μm or 0.45 μm) and dispensed into sterile glass vials. Store the sealed vial in the refrigerator or at room temperature protected from light.
[0034] The HPLC method was used to test the content of idebenone in the preparation.
[0035] The o / w emulsions loaded with idebenone of Examples 2-10 were prepared in a simil...
Embodiment 11
[0040] The idebenone-loaded emulsions of Examples 11-16 with increased oil phase content were prepared by high pressure homogenization, or by idebenone in a mixture of oil, surfactant and stabilizer after addition to the aqueous phase The solution was prepared by spontaneous emulsification without a homogenization step. For Example 11, idebenone was dissolved in acetylated monoglycerides (Myvacet TM 9-45K) and slightly heated (50-55°C) in an oily mixture of D-alpha-tocopheryl polyethylene glycol 1000 succinate (D-alpha tocopheryl polyethyleneglycol 1000succinate, vitamin E (TPGS) surfactant and soy lecithin. Add propylene glycol to the warm solution, then add the water phase heated to 65-70°C and mix with the oily composition at low speed using a propeller mixer to avoid foaming. Examples 13 and 15 were prepared in the same manner as Example 11, while Examples 12, 14 and 16 were treated with a high pressure homogenizer. The formed oil-in-water emulsion was passed through a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com