Idebenone nanometer lipid carrier transdermal absorption preparation and preparation method thereof
A nano-lipid carrier and transdermal absorption preparation technology, which is applied in the field of idebenone nano-lipid carrier transdermal absorption preparation and preparation, can solve problems such as differences in technical parameters, achieve good skin compatibility, avoid residue, The effect of ensuring safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Material
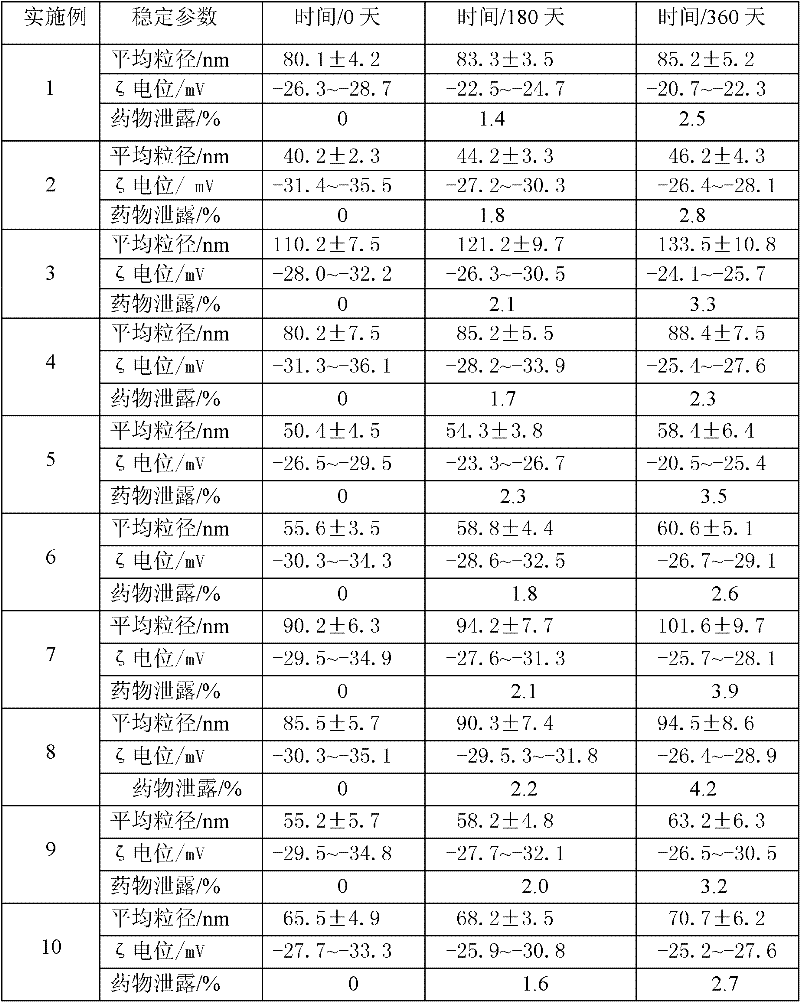
[0049] Weigh the prescribed amount of idebenone, glyceryl behenate, caprylic triglyceride, capric triglyceride and poloxamer-188 and mix evenly, heat to 80°C to form a lipid phase; Sodium amphoteric acetate and ultrapure water are mixed evenly at 80°C to form a water phase; under stirring, the lipid phase is slowly added to the water phase at a temperature of 80°C and mixed, and at a temperature of 80°C, it is emulsified at a high speed of 7000r / min Uniformly shear for 2 minutes for preliminary emulsification, and then use an ultrasonic breaker to perform ultrasonic crushing with a power of 200w for 2 minutes; after the above 4 cycles of high-speed milk uniform shearing and ultrasonic crushing, the mixture is gradually cooled to room temperature, and the loaded moxa powder is prepared. Nanostructured Lipid Carriers of Debenzoquinones. The particle diameter of the carrier is 80.1±4.2nm, the encapsulation efficiency is 90.2%-93.4%, and the ζ potentia...
Embodiment 2
[0051] Material
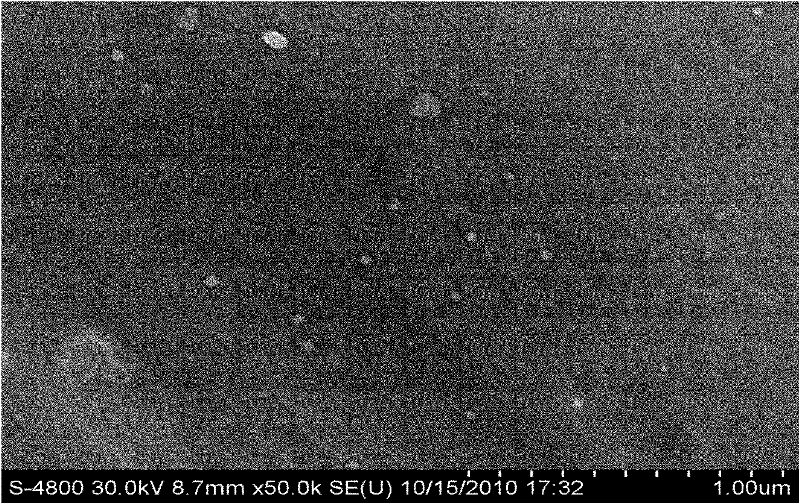
[0052] Weigh the prescribed amount of idebenone, glyceryl palmitostearate, squalene and Tween-80 and mix evenly, heat to 70°C to form a lipid phase; mix the prescribed amount of molecularly distilled monoglyceride with ultrapure water Mix evenly at 70°C to form a water phase; under stirring, slowly add the fat phase to the water phase at 70°C and mix, and at 70°C, perform preliminary emulsification with 7000r / min high-speed milk shear for 2min, Afterwards, the primary emulsion was homogenized with a high-pressure homogenizer, the pressure was 400 bar, and the temperature was controlled at 75°C; after 2 cycles, the sample was poured into a silanized glass container and cooled at room temperature to prepare the loaded idebenne Quinone Nanostructured Lipid Carriers (see figure 2 ). The particle size is 40.2±2.3nm, the encapsulation efficiency is 98.3%~99.1%, and the zeta potential is -31.4~-35.5mV. Then add the prescribed amount of pharmaceutically...
Embodiment 3
[0054] Material
[0055] Weigh the prescribed amount of idebenone, cetyl palmitate, caprylic triglyceride, capric triglyceride and lecithin and mix evenly, heat to 90°C to form a lipid phase; Pure water is mixed evenly at 90°C to form a water phase; under stirring, the lipid phase is slowly added to the water phase at 90°C and mixed, and at 90°C, the high-speed emulsion shearing at 13000r / min for 8min is used for preliminary Emulsification, and then ultrasonic crushing with 200w power for 8 minutes; after the above three cycles of high-speed emulsion shearing and ultrasonic crushing, the mixture is gradually cooled to room temperature, and the nanostructure loaded with idebenone is made lipid carrier (see figure 1 ). The average particle size of the carrier is 110.2±7.5nm, the encapsulation rate is 80.5%-85.7%, and the zeta potential is -28.0-32.2mV. Then add the prescribed amount of pharmaceutically acceptable auxiliary materials to the system to obtain the idebe...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com