Method for preparing idebenone
A technology of idebenone and methyl phenol, applied in the field of preparation of idebenone, can solve the problems of low process yield, high cost, danger and the like, and achieve the effects of good safety, little pollution and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthetic method of idebenone according to the present embodiment comprises the following steps:
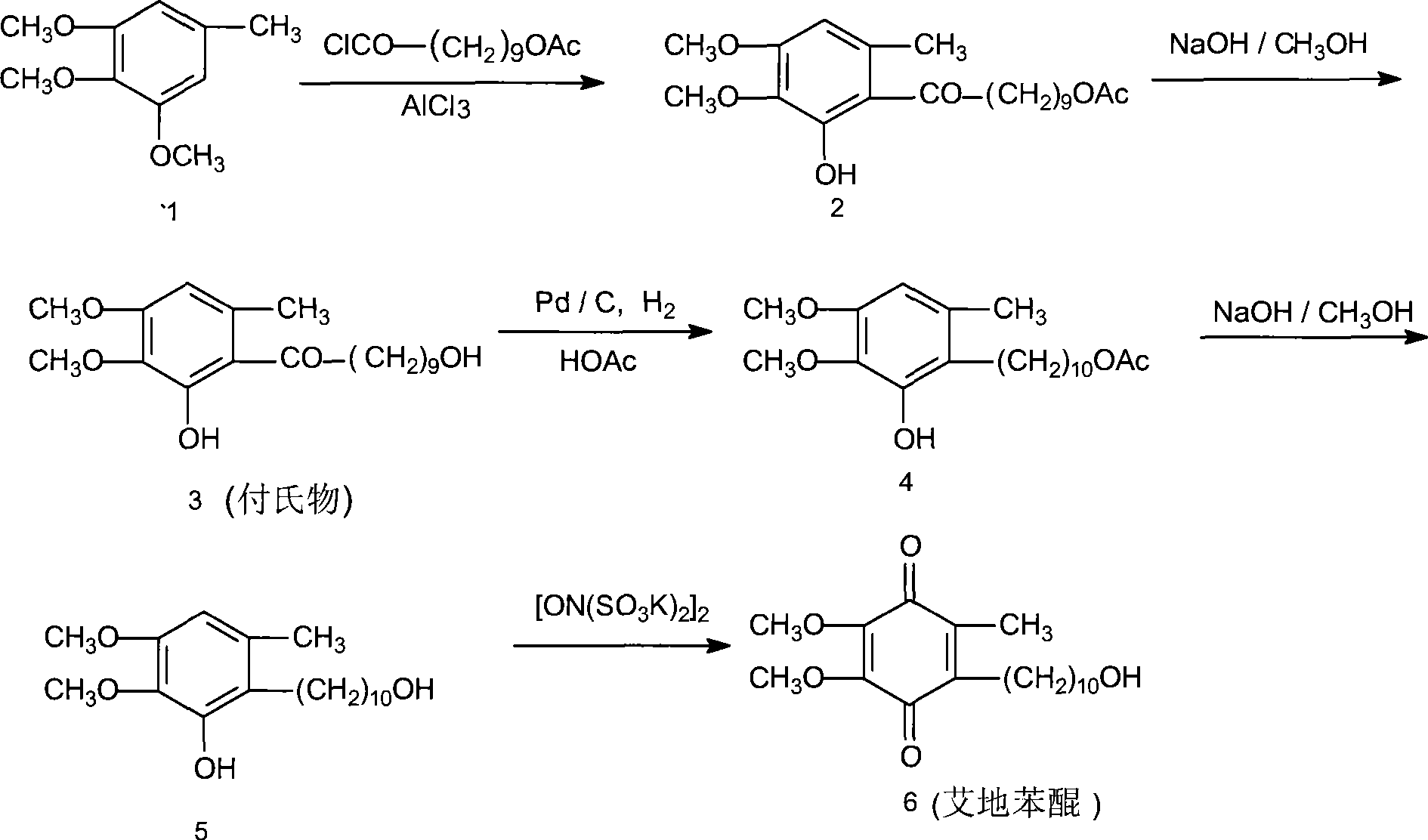
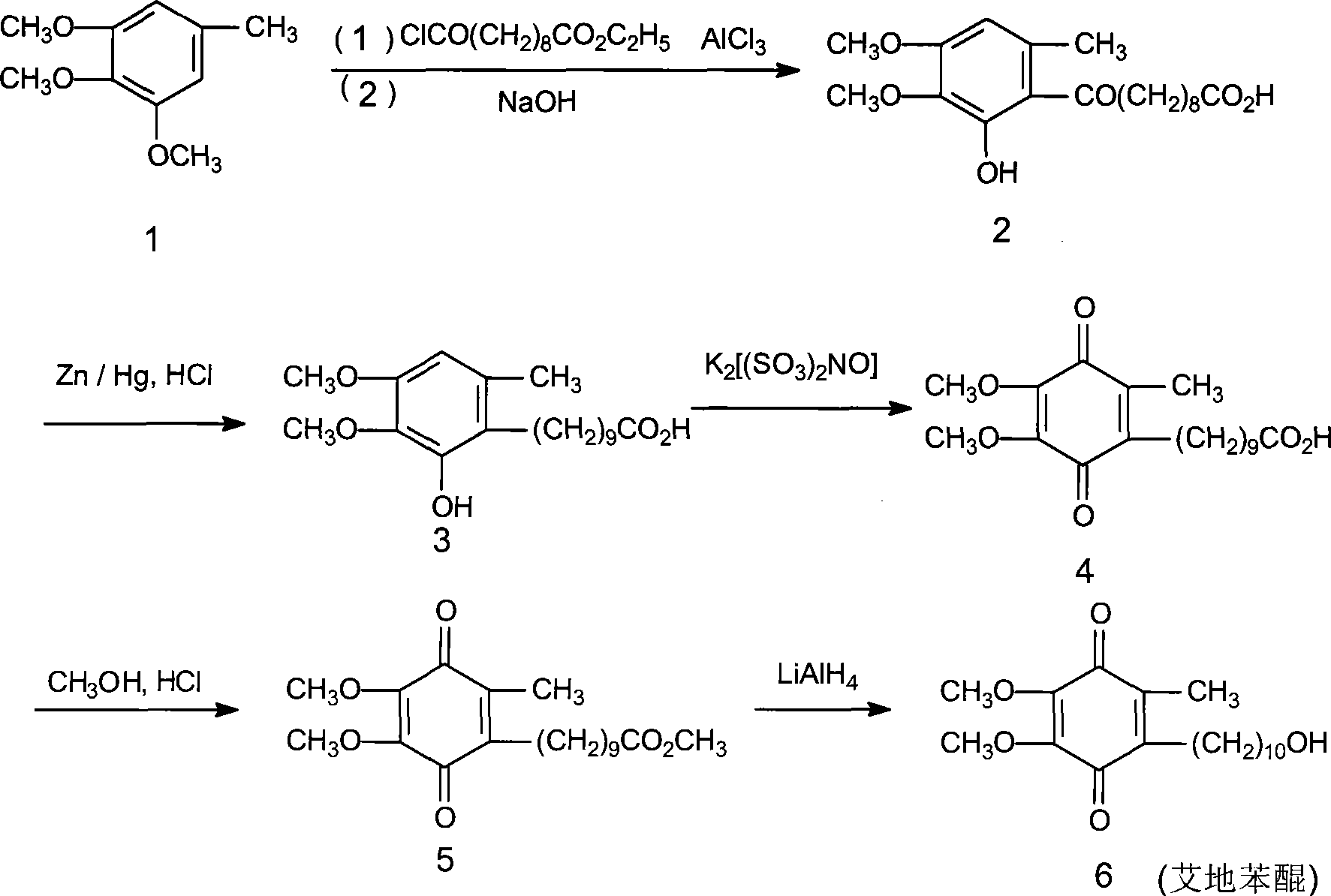
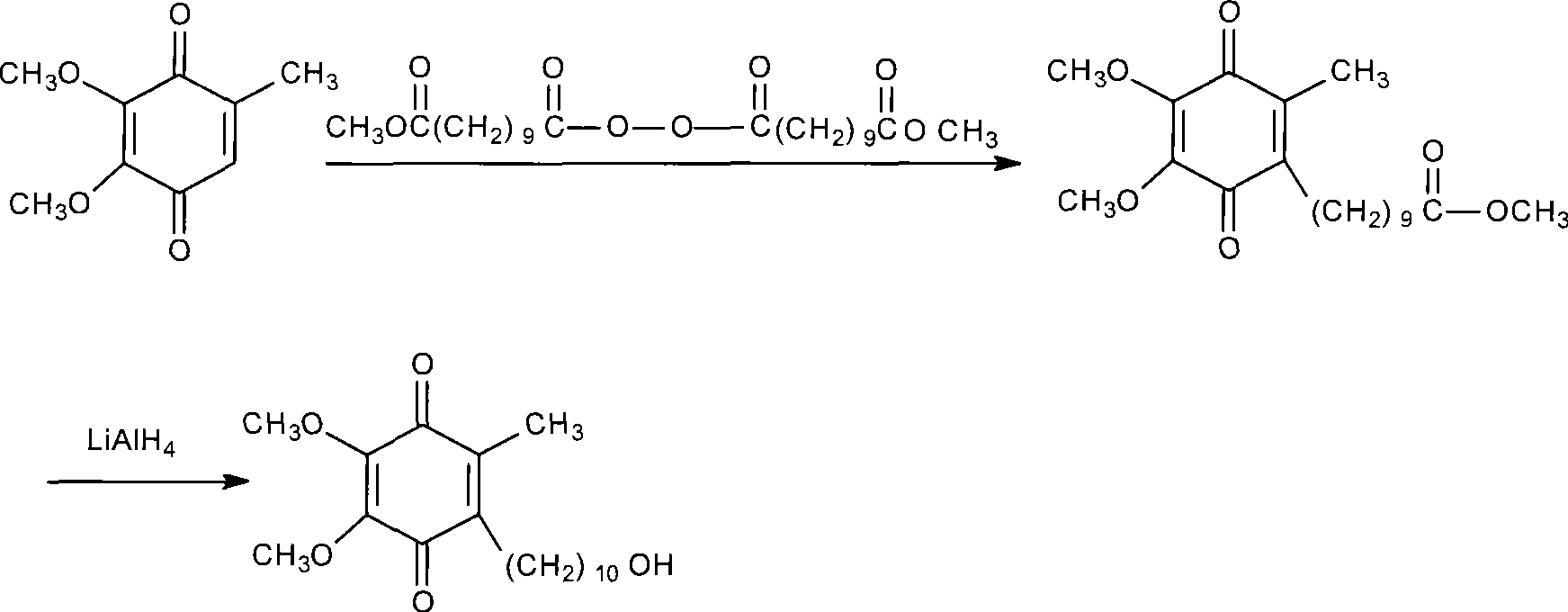
[0029] (1), preparation of 6-(10-acetoxy-1-oxodecyl)-2,3-dimethoxy-5-methylphenol
[0030] Install a mechanical stirrer and a thermometer in a dry and clean 5L reaction flask, and add 2000ml of 1,2-dichloroethane, 182g (about 1mol) of 3,4,5-trimethoxytoluene, and 10-acetoxydecanoyl chloride under stirring. 273g (about 1.5mol), then add 333g (about 2.5mol) of fresh anhydrous aluminum trichloride powder, start the reaction after cooling down to 5°C in an ice-water bath, control the temperature at 0-5°C, after 4 days of reaction, the reaction ends, pour Put into 5000ml of ice water, separate the organic layer, then add 2000ml of 1,2-dichloroethane to extract, combine the organic layers, wash with water three times, 1000ml each time; finally, dry with anhydrous sodium sulfate, decolorize with activated carbon, and evaporate the solvent , to obtain 310 g of a colorless oil...
Embodiment 2
[0038] The synthetic method of idebenone according to the present embodiment comprises the following steps:
[0039] (1), preparation of 6-(10-acetoxy-1-oxodecyl)-2,3-dimethoxy-5-methylphenol
[0040] Install a mechanical stirrer and a thermometer in a dry and clean 5L reaction flask, and add 2000ml of 1,2-dichloroethane, 182g (about 1mol) of 3,4,5-trimethoxytoluene, and 10-acetoxydecanoyl chloride under stirring. 273g (about 1.5mol), then add 390g (about 2.5mol) of fresh anhydrous aluminum triacetate powder, start the reaction after cooling down to 5°C in an ice-water bath, control the temperature at 0-5°C, and finish the reaction after 4 days of reaction, pour into Separate the organic layer in 5000ml of ice water, then add 2000ml of 1,2-dichloroethane for extraction, combine the organic layers, wash with water 3 times, 1000ml each time, dry the organic layer with anhydrous sodium sulfate, decolorize with activated carbon, evaporate the solvent, 335 g (0.88 mol, yield 88.0%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com