Method for catalytically synthesizing di-2-ethyhexyl carbonate by alkali ionic liquid
A technology of diisooctyl carbonate and synthesis method, which is applied in the direction of organic carbonate preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems such as no report of diisooctyl carbonate, and achieve high selectivity , simple operation process and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
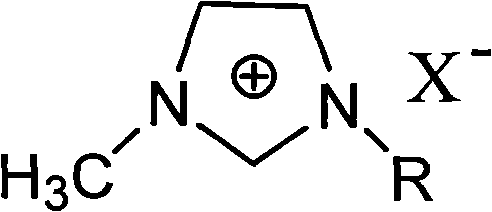
[0020] In a 250mL four-necked flask equipped with a stirrer, a thermometer, a condenser, and a constant pressure dropping funnel, add 64.37g of isooctyl alcohol and 0.86g of 1-ethyl-3-methylimidazolium chloride, heat up to 60°C, and slowly Add 22.26 g of dimethyl carbonate dropwise at a constant speed, and drop it for about 1 hour. Keep the reaction at 60°C for 9 hours, and then connect to a water separator to distill out the methanol and unreacted dimethyl carbonate generated by the reaction. Then, the temperature was raised to 110° C. for 3 hours, and after the reaction was completed, the mixture was cooled and distilled under reduced pressure, and allowed to stand and separate into layers. The upper liquid was distilled under reduced pressure at 3 mmHg to collect a colorless liquid at 170°C to obtain 39.60 g of the product with a yield of 55.92%.
Embodiment 2
[0022] In a 250mL four-neck flask equipped with a stirrer, a thermometer, a condenser, and a constant pressure dropping funnel, add 124.04g of isooctyl alcohol and 2.91g of 1-butyl-3-methylimidazolium imidazolium salt, heat up to 70°C, and slowly Add 21.45g of dimethyl carbonate dropwise at a constant speed, and drop it for about 1 hour. After keeping at 70°C for 8 hours, connect the water separator to distill out the methanol and unreacted dimethyl carbonate generated by the reaction. Then, the temperature was raised to 140° C. for 4 hours, and after the reaction was completed, it was cooled and distilled under reduced pressure, and allowed to stand for stratification. The viscous liquid in the lower layer is 1-butyl-3-methylimidazolium imidazolium salt which can be used twice. The upper liquid was distilled under reduced pressure at 6mmHg to collect a colorless liquid at 180°C to obtain 49.28g of the product with a yield of 72.23 %.
Embodiment 3
[0024] In a 250mL four-neck flask equipped with a stirrer, a thermometer, a condenser, and a constant pressure dropping funnel, add 90.20g of isooctyl alcohol and 2.23g of 1-ethyl-3-methylimidazole hydroxide, and heat up to 80°C , Slowly add 21.42g of dimethyl carbonate dropwise at a uniform speed, and drop it for about 1 hour. Keep the reaction at 80°C for 6 hours and then connect to the water separator to distill out the methanol and unreacted dimethyl carbonate generated by the reaction. Then the temperature was raised to 100° C. for 5 hours, and after the reaction was completed, the mixture was cooled and distilled under reduced pressure, and allowed to stand for stratification. The viscous liquid in the lower layer is 1-ethyl-3-methylimidazole hydroxide which can be used twice. The upper liquid is distilled under reduced pressure at 10 mmHg to collect a colorless liquid at 240 ° C to obtain 40.94 g of the product. The yield is 49.09%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com