Hexa-aromatic heterocyclic diamine and preparation method thereof
A technology for aromatic heterocycle and diazepine, which is applied in the field of six-membered aromatic heterocyclic diamine and its preparation, and can solve the problems of low yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Example 1: Add m-nitroacetophenone (16.5g, 0.1mol), N,N,N',N'-tetramethyl-3-nitro-1,5-diazapentane to the reaction vessel Diene tetrafluoroborate (25.9 g, 0.1 mol) and 64.8 g dimethylformamide. Under mechanical stirring, potassium tert-butoxide (22.4 g, 0.2 mol) was added and stirred at 40° C. for 2 hours. Then, ammonium acetate (46.2 g, 0.6 mol) and glacial acetic acid (36.0 g, 0.6 mol) were added, and the temperature was raised to 100° C. to react for 6 hours. Cool to room temperature and filter off the solid. The solid was washed with water and ethanol and dried to obtain 2-(3-nitrophenyl)-5-nitropyridine;
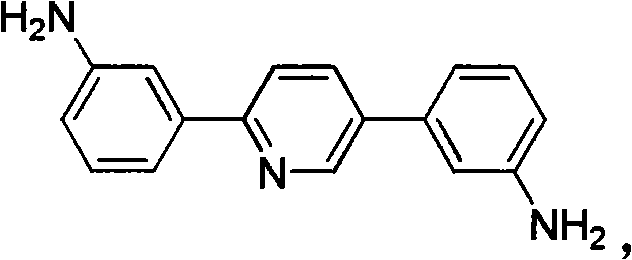
[0064] The 2-(3-nitrophenyl)-5-nitropyridine obtained above was added to 16.5 g of ethanol, 0.165 g of palladium on carbon and hydrazine hydrate (5.0 g, 0.1 mol) were added, and heated to reflux for 6 hours. The palladium carbon was filtered out while it was hot, and after the filtrate was cooled to room temperature, the product was precipitated. After filtra...
Embodiment 2
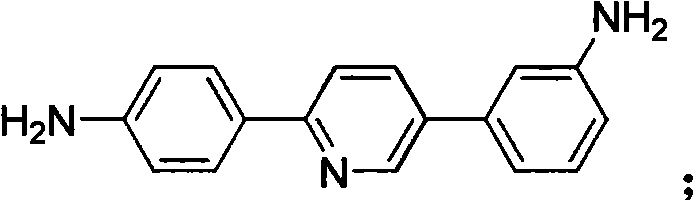
[0065] Example 2: Add m-nitroacetophenone (16.5g, 0.1mol), N,N,N',N'-tetrabutyl-3-(3-nitrophenyl)-1 to the reaction vessel, 5-diazapentadiene perchloric acid (51.6 g, 0.1 mol) and 421 g dimethylacetamide solvent. Under mechanical stirring, sodium methoxide (16.2 g, 0.3 mol) was added and stirred at 10° C. for 4 hours. Then, ammonium acetate (7.7 g, 0.1 mol) and glacial acetic acid (60.0 g, 1.0 mol) were added, and the temperature was raised to 110° C. for 1 hour. Cool to room temperature and filter off the solid. The solid was washed with water and ethanol and dried to obtain 2,5-bis[(3-nitrophenyl)]pyridine, which was directly used in the next step;
[0066] Add the 2,5-bis[(3-nitrophenyl)]pyridine obtained above into 165 g of ethanol, add 1.65 g of palladium on carbon, and hydrazine hydrate (50.0 g, 1.0 mol), and heat to reflux for 10 hours. The palladium carbon was filtered out while it was hot, and after the filtrate was cooled to room temperature, the product was preci...
Embodiment 3
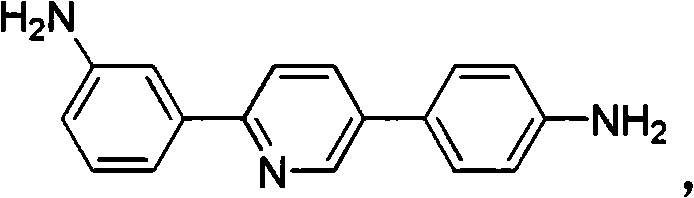
[0067] Example 3: Add m-nitroacetophenone (16.5g, 0.1mol), N,N,N',N'-tetraoctyl-3-(4-nitrophenyl)-1 to the reaction vessel, 5-diazapentadiene perchlorate (74.0 g, 0.1 mol) and 1353 g isopropanol. Under mechanical stirring, potassium tert-butoxide (44.8 g, 0.4 mol) was added, and stirred at 60° C. for 1 hour. Then, ammonium acetate (77.0 g, 1.0 mol) and glacial acetic acid (6.0 g, 0.1 mol) were added, and the temperature was raised to 80° C. for 10 hours. Cool to room temperature and filter off the solid. The solid was washed with water and ethanol and dried to obtain 2-(3-nitrophenyl)-5-(4-nitrophenyl)pyridine, which was directly used in the next step;
[0068] Add 2-(3-nitrophenyl)-5-(4-nitrophenyl)pyridine obtained above into 80 g of ethanol, add 0.825 g of palladium carbon, hydrazine hydrate (15.0 g, 0.3 mol), and heat to reflux for 5 Hour. The palladium carbon was filtered out while it was hot, and after the filtrate was cooled to room temperature, the product was prec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com