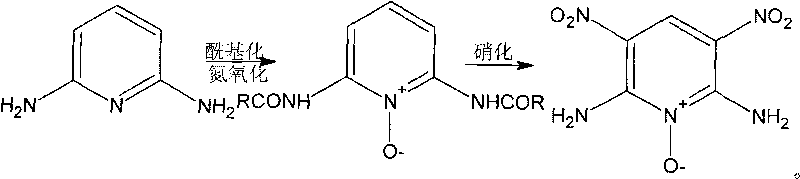
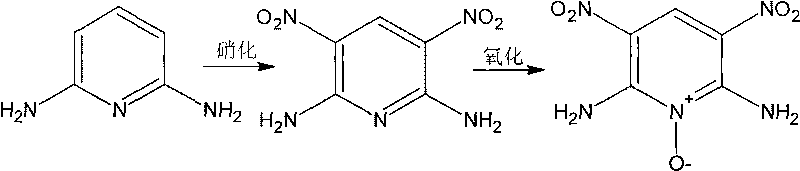
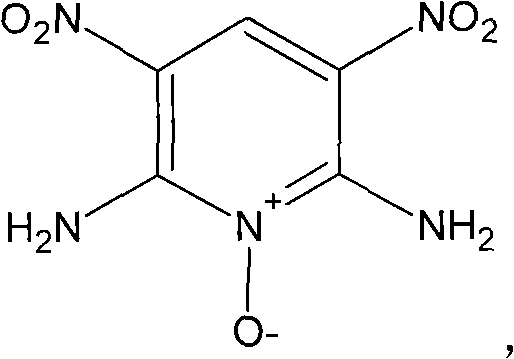
Preparation method of high-energy insensitive explosive 2,6-diamino-3,5-dinitro pyridine-1-oxide
A technology of dinitropyridine and diaminopyridine, applied in the field of explosive synthesis, can solve the problems of difficulty in realizing industrialization, multiple impurities, high cost, etc., and achieve the effects of simple product post-processing, simplified process flow, and reduced production cost.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] For the synthesis of 2,6-diacetylaminopyridine-1-oxide, the synthetic route is shown in accompanying drawing II. In a 100mL three-neck flask, add 5.45g (0.05mol) of 2,6-diaminopyridine, and add 33g of acetic anhydride dropwise while stirring; heat up to 45°C and continue the reaction for 1.5h. TLC detects that the basic reaction of the raw materials is complete, stop the reaction, and stir evenly . In the acetylation reaction solution, 0.079mol40% peracetic acid was added dropwise, reacted at 45°C for 2h, TLC detected that the raw materials had basically reacted completely, stopped the reaction, concentrated under reduced pressure, extracted three times with chloroform, combined organic phases and distilled to obtain 2,6- Diacetylaminopyridine nitrogen oxide crude product, yield 92%.
Embodiment 2
[0037] For the synthesis of 2,6-diacetylaminopyridine-1-oxide, the synthetic route is shown in accompanying drawing II. In a 100mL three-neck flask, add 5.45g (0.05mol) of 2,6-diaminopyridine, and add 33g of acetic anhydride dropwise while stirring; heat up to 45°C and continue the reaction for 1.5h. TLC detects that the basic reaction of the raw materials is complete, stop the reaction, and stir evenly . In the acetylation reaction solution, add 0.079mol40% peracetic acid and 0.5ml concentrated sulfuric acid dropwise, react at 45°C for 2h, TLC detects that the raw materials have basically reacted completely, stop the reaction, concentrate under reduced pressure, extract three times with chloroform, combine the organic phases and distill The crude product of 2,6-diacetylaminopyridine nitrogen oxide was obtained with a yield of 90%.
Embodiment 3
[0039] For the synthesis of 2,6-diacetylaminopyridine-1-oxide, the synthetic route is shown in accompanying drawing II. In a 100mL three-neck flask, add 5.45g (0.05mol) of 2,6-diaminopyridine, and add 33g of acetic anhydride dropwise while stirring; heat up to 45°C and continue the reaction for 1.5h. TLC detects that the basic reaction of the raw materials is complete, stop the reaction, and stir evenly . In the acetylation reaction liquid, add dropwise 0.079mol40% peracetic acid, 0.5ml concentrated sulfuric acid and 0.3g NaHSO 4 , reacted at 45° C. for 2 h, TLC detected that the reaction of the raw materials was basically complete, stopped the reaction, concentrated under reduced pressure, extracted three times with chloroform, combined organic phases and distilled to obtain crude 2,6-diacetylaminopyridine nitrogen oxide with a yield of 91.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com