Compositions and methods for enhancing transmucosal delivery
A composition, oral mucosa technology, applied in the directions of drug combination, drug delivery, pill delivery, etc., can solve problems such as unsatisfactory
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0142] Preparation of the composition of the present invention
[0143]Exemplary solid forms for oromucosal (eg, sublingual and buccal) administration include films, dispersible fluid or semisolid compositions, and solid forms. Solid dosage forms intended for distribution on or over the oral mucosa may conveniently be presented in the form of hard candies or chewable confectionaries. Other forms may include tablets, pills, capsules, powders, and granulated materials for dispersion in the oral cavity. These solid compositions may include pharmaceutically acceptable inert ingredients such as diluents (such as calcium carbonate, sodium chloride, lactose, calcium phosphate, sodium phosphate, and similar diluents); granulating agents and disintegrants (such as potato starch, Alginic acid and similar granulating and disintegrating agents); Binders (such as starch, gelatin, acacia and similar binders); Lubricants (such as magnesium stearate, stearic acid, talc and similar lubrican...
Embodiment 1
[0181] Example 1. Preparation of compositions containing DL-phenylalanine
[0182] A soft buccal dosage formulation was prepared in the form of chocolate chips, each weighing 3 grams.
[0183] Each tablet contains:
[0184] DL-Phenylalanine 150mg
[0185] Methylsulfonylmethane (MSM) 100mg
[0186] Vegetable Oil 30%
[0187] Cocoa Butter 30%
[0188] Sucralose 0.1%
[0190] Lecithin 10%, and
[0191] Add purified water to 100%.
[0192] The method for preparing said composition comprises the following steps:
[0193] Step 1: Pour 20ml of deionized water into the homogenization system and heat to a temperature of 50°C.
[0194] Step 2: Add MSM to water and homogenize well.
[0195] Step 3: Add phenylalanine to water and homogenize well.
[0196] Step 4: Add the sucralose to the water and homogenize well.
[0197] Step 5: Mix the ingredients well for 30 minutes to produce Solution A.
[0198] Step 6: Add lecithin to solution A and mix w...
Embodiment 2
[0203] Example 2. Comparison of the bioavailability of formulations containing DL-phenylalanine in the presence or absence of methylsulfonylmethane (MSM)
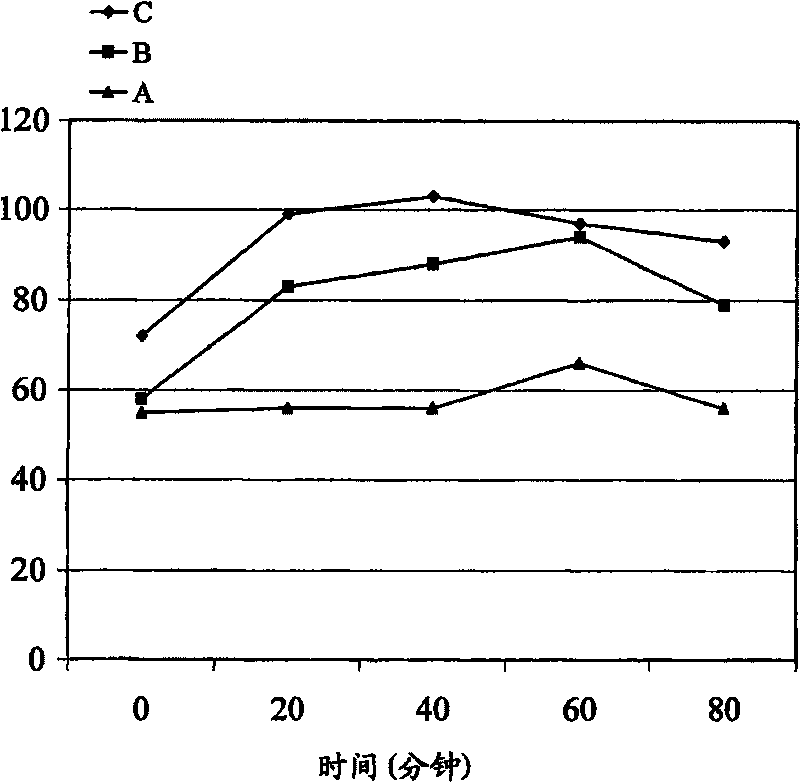
[0204] Tablets prepared by the method described in Example 1 were administered to three healthy adult male subjects at a dose of 450 mg DL-phenylalanine per subject in the oral cavity between the gum and the cheek. (A) MSM-free formulation, 60 second retention time (intraoral); (B) MSM-containing formulation, 30-second retention time (intraoral); and (C) MSM-containing formulation, 60-second retention time (intraoral). These subjects fasted from 12 hours before administration until the end of the test.
[0205] Blood samples (3 ml) were taken before administration and 20, 40, 60 and 80 minutes after administration of the formulation. The determination of DL-phenylalanine in blood was carried out according to the following analytical method.
[0206] After separation of blood, plasma was stored at -20°C until chemical ana...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com