Nasal pharmaceutical composition adopting cyclodextrin to include methylprednisolone aceponate
A technology of methylprednisolone acetin and composition, which is applied in the field of nasal pharmaceutical composition, can solve the problems of poor stability, affecting compliance, and undisclosed issues, and achieve the effects of stable content, good stability, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
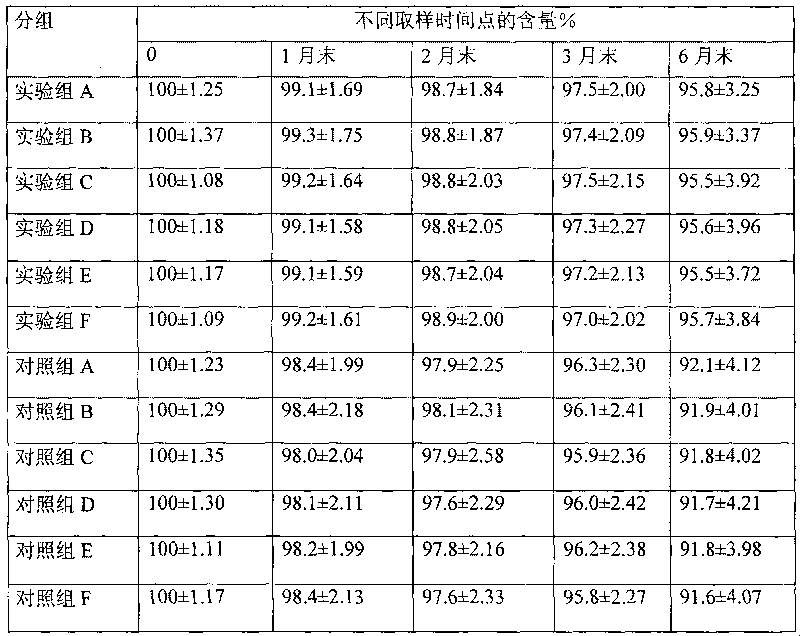
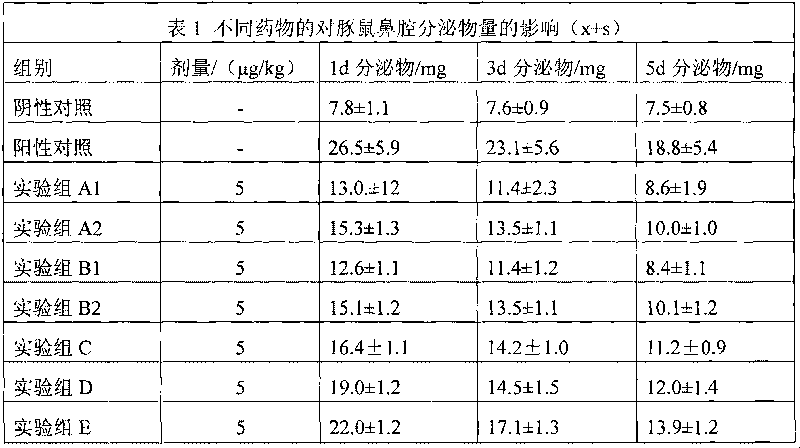
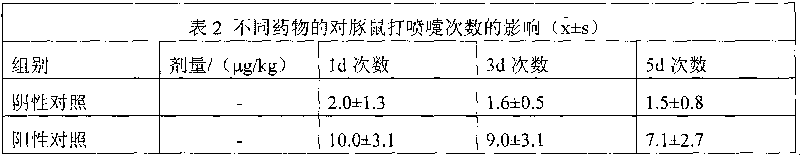
[0049] Dissolve 2 g of 3-hydroxypropyl β-cyclodextrin in 15 ml of 95% ethanol at room temperature, add 0.2 g of methylprednisolone acetate, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220 nm), add 8 ml of Water for injection, stirred for 5 minutes, concentrated under reduced pressure, and evaporated to remove ethanol to obtain an aqueous solution of methylprednisolone acetone / 3-hydroxypropyl β-cyclodextrin inclusion complex, which is set aside.
Embodiment 2
[0051] Dissolve 2g of 2-hydroxypropyl-β-cyclodextrin in 20ml of acetone at 40±2°C, add 0.5g of methylprednisolone acetone, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220nm), Add 3mL of water for injection, stir for 5 minutes, concentrate under reduced pressure, add 4ml of water for injection twice during the concentration process, remove acetone, and obtain dexamethasone acetate / 2-hydroxypropyl-β-cyclodextrin inclusion complex Aqueous solution, spare.
Embodiment 3
[0053] Dissolve 7 g of 2-hydroxyethyl-β-cyclodextrin in 20 ml of absolute ethanol at room temperature, add 0.5 g of methylprednisolone acetate, stir to dissolve, stir for another 30 minutes, filter through a microporous membrane (220 nm), and stir Concentrate under reduced pressure for 5 minutes, add 3.5ml of water for injection three times during the concentration process, and evaporate ethanol to obtain methylprednisolone acetone / 2-hydroxyethyl-β-cyclodextrin inclusion aqueous solution, which is set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com