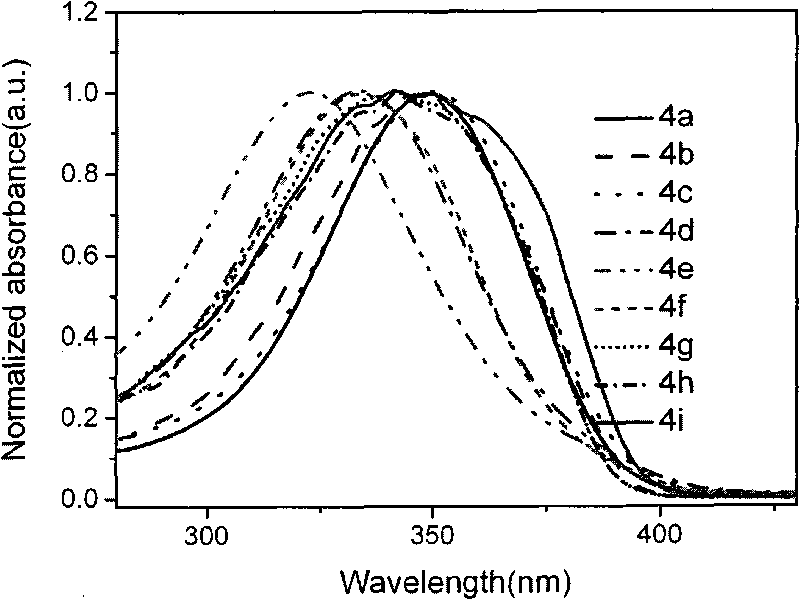
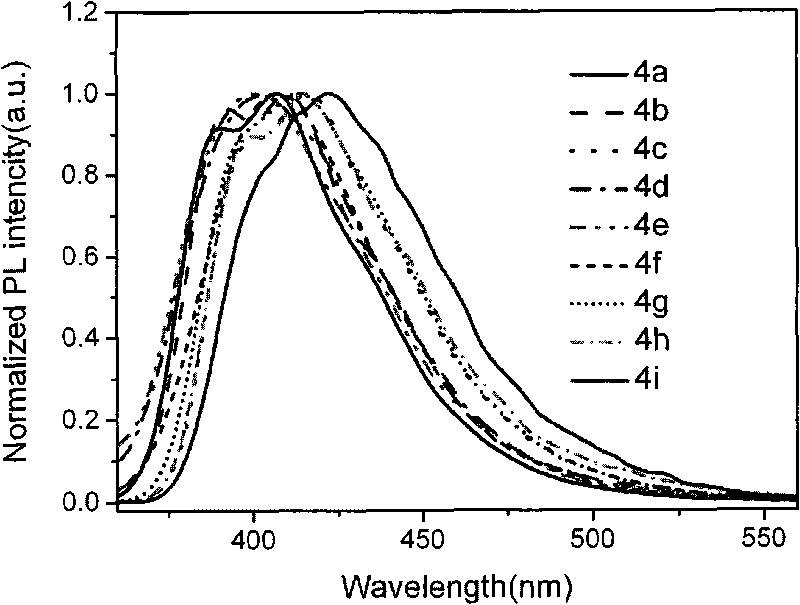
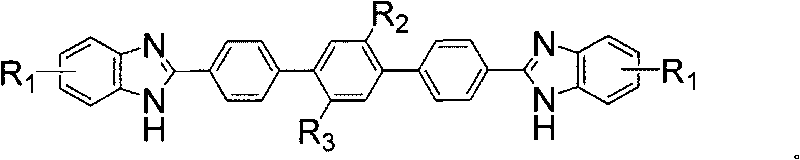
Terphenyl bridged double-benzimidazoles compound as well as synthesis method and application thereof
A bibenzimidazole and terphenyl bridge technology, applied in the field of optoelectronic materials, can solve the problems of insufficient stability and poor color purity of blue light materials, achieve the effect of improving electron transport ability and luminescent performance, and changing chemical characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the synthesis of 4,4 "-dicyano-p-terphenyl
[0029] Add 3.23g (22mmol) of 4-cyanophenylboronic acid, 2.36g (10mmol) of p-dibromobenzene, 9.32g (88mmol) of anhydrous sodium carbonate, and 0.462 g (0.66mmol) and 50mL tetrahydrofuran, heated to 50-65°C, and reacted at this temperature for 20 hours. Remove tetrahydrofuran under reduced pressure, add 30mL of dichloromethane to dissolve, wash with water, and separate layers. After the organic layer is dried over anhydrous sodium sulfate, part of the dichloromethane is concentrated, the temperature is lowered to precipitate a white solid, and the white crystal powder is obtained by suction filtration and drying. 4,4" -Dicyano-p-terphenyl 2.03g, yield 72.4%, melting point 288-290°C.
Embodiment 2
[0030] Embodiment 2: the synthesis of 2'-methyl-4,4 "-dicyano-p-terphenyl
[0031] Add 3.38g (23mmol) of 4-cyanophenylboronic acid, 2.50g (10mmol) of 2,5-dibromotoluene, 12.12g (88mmol) of anhydrous potassium carbonate, bis(triphenylphosphine) dichloro Palladium chloride 0.462g (0.66mmol) and 50mL tetrahydrofuran were heated up to 50-65°C and reacted at this temperature for 15 hours. Remove tetrahydrofuran under reduced pressure, add 30 mL of dichloromethane to dissolve, wash with water, separate layers, dry the oil layer over anhydrous sodium sulfate, concentrate part of the dichloromethane, cool down to precipitate white crystals. After suction filtration and drying, 2.26 g of white crystal 2'-methyl-4,4"-dicyano-p-terphenyl was obtained, with a yield of 76.8% and a melting point of 254-255°C. 1 H NMR (CDCl 3 , 500MHz): δ7.77-7.70(m, 6H), 7.52-7.45(m, 4H), 7.31(d, 1H), 2.34(s, 3H); 13 C NMR (CDCl 3 , 300MHz): δ145.98, 145.02, 140.44, 139.20, 136.05, 132.69, 132.14, 130.3...
Embodiment 3
[0032] Embodiment 3: 2'-nitro-4,4 "-the synthesis of dicyano-p-terphenyl
[0033] Add 2.94g (20mmol) of 4-cyanophenylboronic acid, 2.81g (10mmol) of 2-nitro-1,4-dibromobenzene, 9.32g (88mmol) of anhydrous sodium carbonate, tetrakis (triphenyl phosphine) palladium 0.762g (0.66mmol), and 50mL tetrahydrofuran, the temperature was raised to 55-65°C, and the reaction was carried out at this temperature for 18 hours. Tetrahydrofuran was removed under reduced pressure, dissolved in 30 mL of dichloromethane, washed with water, and separated into layers. The organic layer was dried over anhydrous sodium sulfate, concentrated, and the temperature was lowered to precipitate pale yellow crystals. After suction filtration and drying, 2.23 g of yellow crystal powder 2'-nitro-4,4"-dicyano-p-terphenyl was obtained, with a yield of 68.8% and a melting point of 235-237°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com