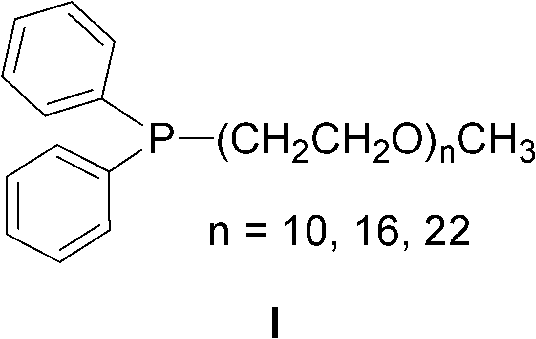Method for preparing biaryl compound in pure water
A pure aqueous solution and compound technology, applied in the field of preparing biaryl compounds, can solve the problems of low reactivity, poor selectivity, large amount of catalyst, etc., and achieve the effect of wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] The preparation of embodiment 1 4-phenyl anisole
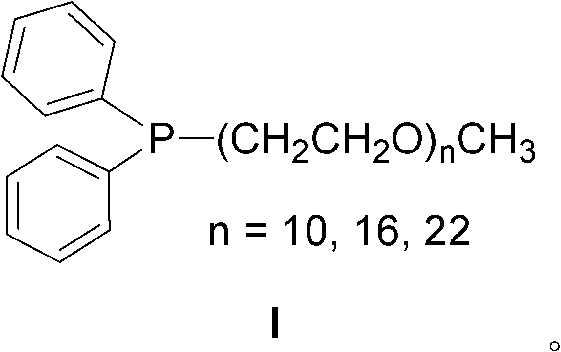
[0014] Under the protection of nitrogen, first complex palladium chloride (0.005mmol), ligand I (n=22, 0.005mmol) and deoxygenated water (1ml) in a Schlink bottle for 30 minutes, and then add triethylamine (1.0mmol ), 4-bromoanisole (0.5mmol), phenylboronic acid (0.75mmol), reacted for 10 minutes under magnetic stirring at 100°C, and followed the reaction by thin-layer chromatography. After the reaction, add 2ml ether to the reaction mixture for extraction, stand at room temperature and separate the phases, add 15mL saturated brine to the ether phase and extract the reaction product with ethyl acetate (15mL×3), combine the organic phases, anhydrous Na 2 SO 4 Dry, filter, and use a rotary evaporator to concentrate to obtain a crude product. Column chromatography obtains the target product. The eluent used in column chromatography is petroleum ether, and the product structure passes through 1 H NMR and mass spectral ide...
Embodiment 2
[0015] The preparation of embodiment 2 3-phenyl anisole
[0016] Under the protection of nitrogen, first complex palladium chloride (0.005mmol), ligand I (n=22, 0.01mmol) and deoxygenated water (1ml) in a Schlink bottle for 30 minutes, and then add sodium hydroxide (1.0mmol ), 3-bromoanisole (0.5mmol), phenylboronic acid (0.75mmol), reacted under magnetic stirring at 100°C for 30 minutes, and followed the reaction by thin-layer chromatography. After the reaction, add 2ml of ether to the reaction mixture for extraction, stand at room temperature for phase separation, add 15ml of saturated brine to the ether phase and extract the reaction product with ethyl acetate (15ml×3), combine the organic phases, anhydrous Na 2 SO 4 Dry, filter, and use a rotary evaporator to concentrate to obtain a crude product. Column chromatography obtains the target product. The eluent used in column chromatography is petroleum ether, and the product structure passes through 1 H NMR and mass spectra...
Embodiment 3
[0017] The preparation of embodiment 3 2-phenyl anisole
[0018] Under the protection of nitrogen, first complex palladium acetate (0.005mmol), ligand I (n=22, 0.01mmol) and deoxygenated water (1ml) in a Schlink bottle for 30 minutes, and then add triethylamine (1.0mmol) sequentially , 2-bromoanisole (0.5mmol), phenylboronic acid (0.75mmol), reacted under magnetic stirring at 100°C for 30 minutes, and tracked the reaction by thin-layer chromatography. After the reaction, add 2ml of ether to the reaction mixture for extraction, stand at room temperature for phase separation, add 15ml of saturated brine to the ether phase and extract the reaction product with ethyl acetate (15ml×3), combine the organic phases, anhydrous Na 2 SO 4 Dry, filter, and use a rotary evaporator to concentrate to obtain a crude product. Column chromatography obtains the target product. The eluent used in column chromatography is petroleum ether, and the product structure passes through 1 H NMR and mass...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com