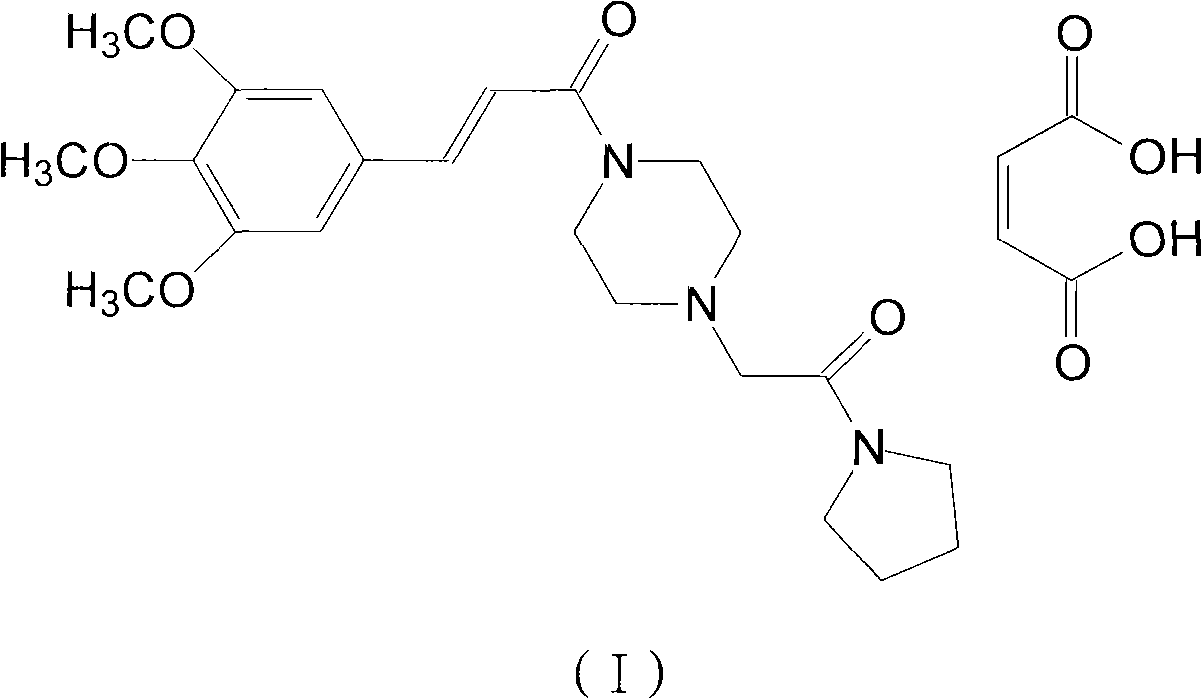
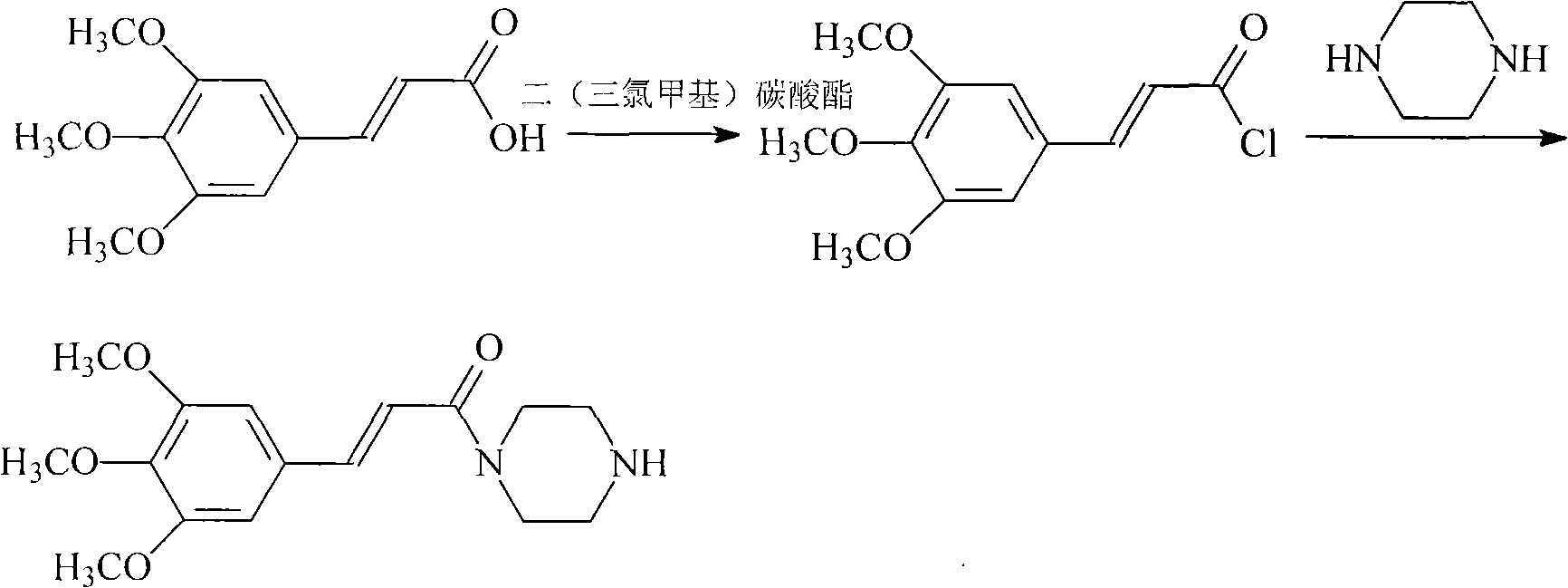
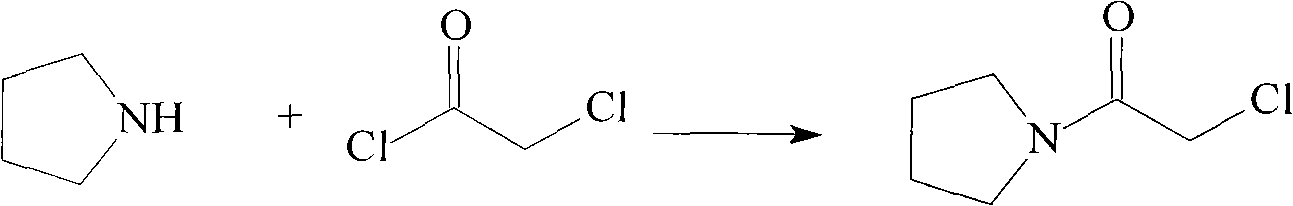
Cinepazide maleate compound with novel route
A kind of technology of cinepazide maleate and compound, which can be applied in the field of medicine and can solve the problems of strong toxicity of solvent anhydrous benzene and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation of embodiment 1 cinepazide maleate
[0048](1) Add 22.2g of chloroacetyl chloride into 90mL of chloroform, stir and cool down to -10°C, slowly add 36ml of a chloroform solution of 14g tetrahydropyrrole and 21g triethylamine dropwise, during which the temperature is not higher than 0°C, and the dropwise Afterwards, stir to return to room temperature, continue to react for 1 hour, add water to wash three times, 30ml each time, dry with anhydrous sodium sulfate, distill off the solvent to obtain a brown liquid, place it at room temperature to solidify, and obtain 24g of the product;
[0049] (2) Suspend 9.6 g of 3,4,5-trimethoxycinnamic acid in 30 g of dichloromethane, add 30 g of bis(trichloromethyl) carbonate under stirring, stir at room temperature for 3 hours, then distill under reduced pressure Chloromethane, under ice-cooling, add piperazine 8.2g and 25% potassium hydroxide aqueous solution 50ml, stir at 0°C for 2 hours, solid precipitates, filter to ...
Embodiment 2
[0052] The preparation of embodiment 2 cinepazide maleate
[0053] (1) Add 44.3g of chloroacetyl chloride into 200mL of chloroform, stir and cool down to -5°C, slowly add 36ml of a chloroform solution of 28.4g of tetrahydropyrrole and 45g of triethylamine dropwise, during which the temperature is not higher than 0°C, dropwise After completion, stir to return to room temperature, continue to react for 1 hour, add water to wash three times, 50ml each time, dry with anhydrous sodium sulfate, distill off the solvent to obtain a brown liquid, place it at room temperature to solidify, and obtain 48.5g of the product;
[0054] (2) Suspend 19.3 g of 3,4,5-trimethoxycinnamic acid in 60 g of dichloromethane, add 60 g of bis(trichloromethyl) carbonate under stirring, stir at room temperature for 3 hours, then distill under reduced pressure Chloromethane, under ice-cooling, add 16.5g of piperazine and 100ml of 25% aqueous potassium hydroxide solution, stir at 0°C for 2 hours, a solid prec...
Embodiment 3
[0060] The refining of embodiment 3 cinepazide maleate
[0061] Take 10 g of cinepazide maleate salt, add 140 mL of absolute ethanol for recrystallization, and obtain 9.5 g of the product with a yield of 95% and a melting point of 172°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com