Method for preparing fluoro alpha-D-arabinofuranose compound
A technology for furanoses and compounds is applied in the field of preparation of fluoro α-D-arabinofuranoses, and can solve the problems of many by-products, serious environmental pollution, difficulty in industrialized production and the like, and improve the reaction yield. , The effect of high product purity and convenient post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
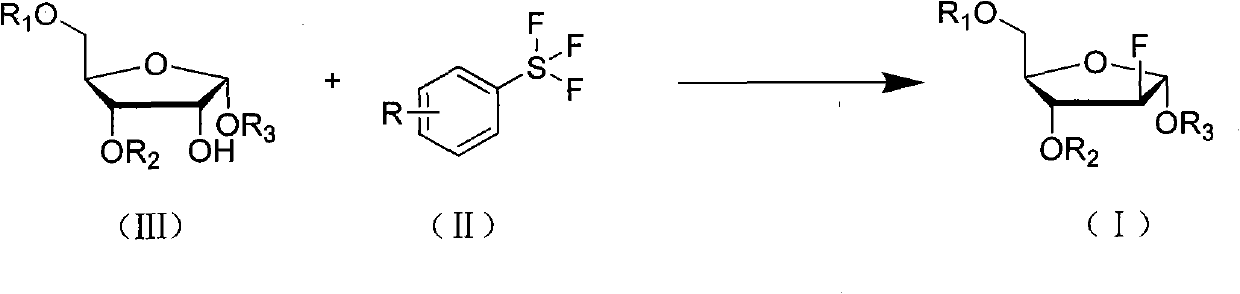
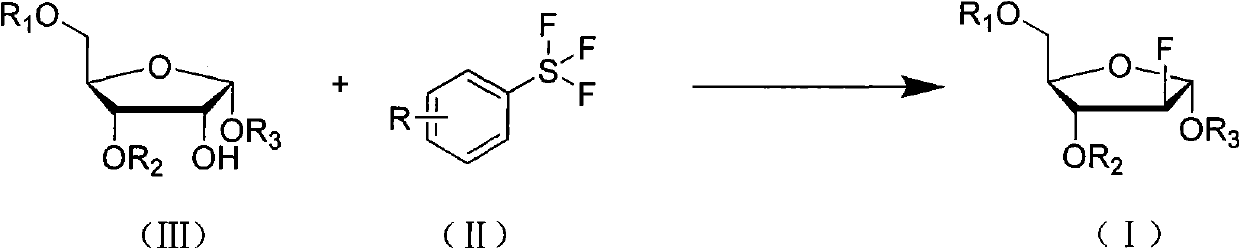
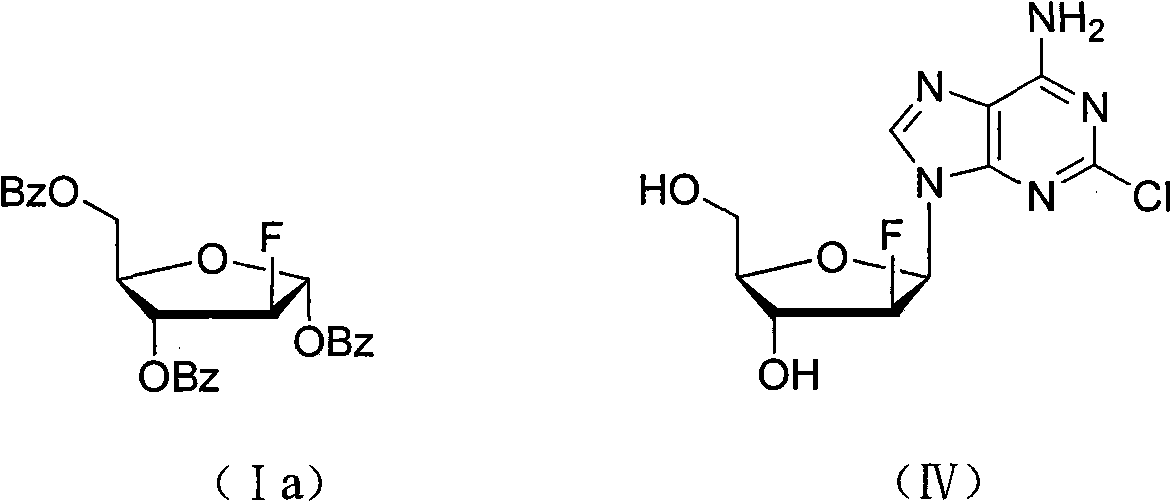
[0028] Under anhydrous and oxygen-free conditions, 4.62g (0.01mol) of 1,3,5-tribenzoyl ribose (compound IIIa) was dissolved in 45mL of dichloromethane, and 4.2g (0.02mol) of 4 - dichloromethane solution of formic acid phenylsulfur trifluoride (compound II-1), after the dropwise addition, rise to room temperature and stir until TLC monitors that the reaction is complete. The reaction solution was poured into ice water, separated, the aqueous layer was extracted twice with dichloromethane, the organic layer was combined, and the organic layer was sequentially washed with saturated NaHCO 3 liquid, water, and saturated brine, and dried over anhydrous sodium sulfate. After filtering and evaporating to dryness, the residue was recrystallized from ethanol to obtain 2.70 g of 2-deoxy-2-fluoro-1,3,5-tribenzoyl-α-D-arabinofuranose (Compound Ia), with a yield of 58%.
[0029] 1 HNMR (300Hz, CDCl 3 ): 4.6-4.8 (m, 3H), 5.3-5.4 (d, J=45.6Hz, 1H), 5.65-5.66 (dd, J 1 =2.94Hz,J...
Embodiment 2
[0033]
[0034] Dissolve 5.04g (0.01mol) of 1,3,5-tri-toluoyl ribose (compound IIIb) in 50mL of toluene, and add 3.36g (0.015mol) of 4-methoxycarbonylphenyl trifluoride dropwise under ice-cooling After the toluene solution of sulfur (compound II-7) was added dropwise, it was raised to room temperature and stirred until the reaction was completed as monitored by TLC. The reaction solution was poured into ice water, separated, the water layer was extracted twice with toluene, the organic layer was combined, and the organic layer was sequentially washed with saturated NaHCO 3 liquid, water, and saturated brine, and dried over anhydrous sodium sulfate. Filtered, evaporated to dryness, and the residue was recrystallized with ethyl acetate to obtain 3.22g of 2-deoxy-2-fluoro-1,3,5-tri-p-toluoyl-α-D-arabinofuranose (compound I b). rate of 64%.
[0035] 19 FNMR (282Hz, CDCl 3 ): -184.3.
Embodiment 3
[0037]
[0038] 2.76g (0.01mol) of 1,3,5-triacetyl ribose (compound IIIc) was dissolved in 30mL of acetonitrile, and 3.82g (0.02mol) of 3-cyanophenylsulfur trifluoride (compound II-11) acetonitrile solution, after the dropwise addition is complete, rise to room temperature and stir until the reaction is complete as monitored by TLC. The solvent was evaporated, and the residue was added to ice water and dichloromethane to separate the layers. The aqueous layer was extracted twice with dichloromethane, and the organic layers were combined. The organic layer was sequentially washed with saturated NaHCO 3 , water, saturated brine, and dried over anhydrous sodium sulfate. Filter, evaporate to dryness, and recrystallize the residue with ethyl acetate to obtain 2.09g of 2-deoxy-2-fluoro-1,3,5-triacetyl-α-D-arabinofuranose (Compound Ic), yield 75% .
[0039] 19 FNMR (282Hz, CDCl 3 ): -190.8.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com