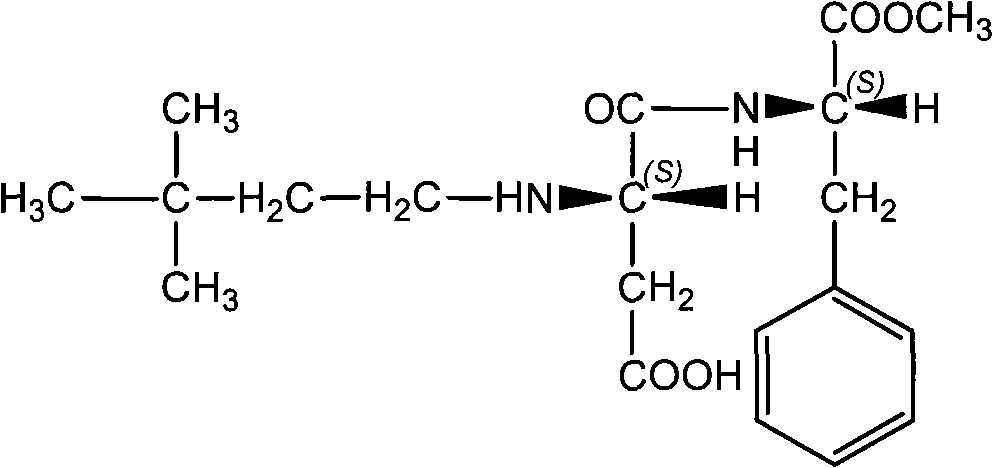
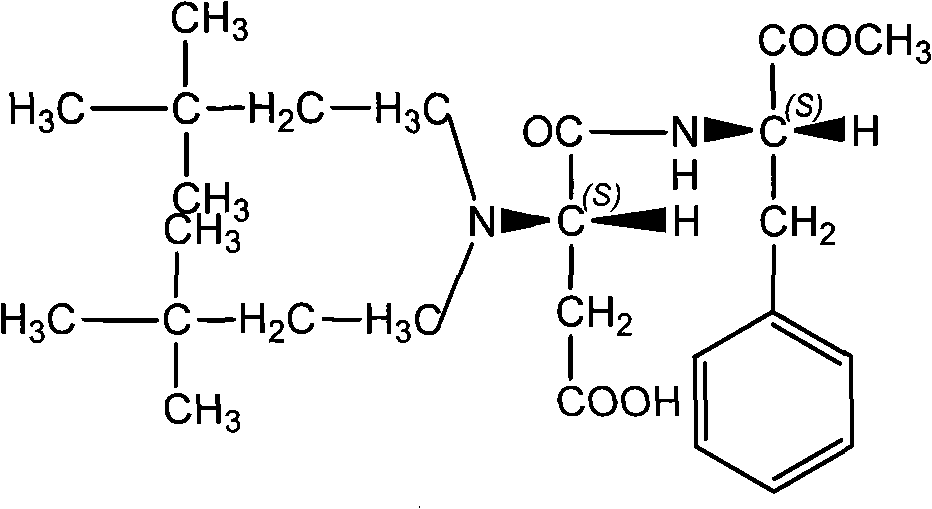
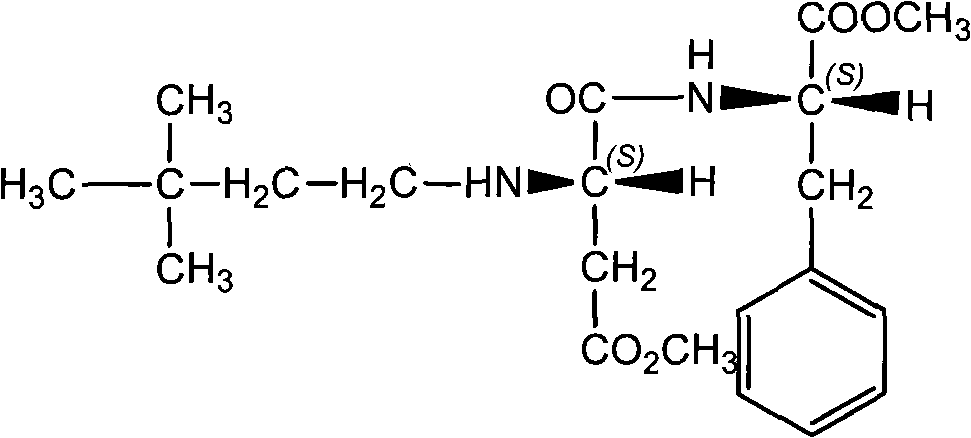
Method for synthesizing neotame
A technology of neotame and aspartame, applied in the direction of peptides, etc., can solve problems such as not being suitable for large-scale production, increasing production costs, and incomplete reaction, and achieve the effects of reducing the difficulty of purification, improving utilization rate, and shortening reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] A method for synthesizing neotame, comprising the steps of:
[0026] (1) In methanol, 3,3-dimethylbutyraldehyde and aspartame with a molar ratio of 1:1 are under the catalysis of 5% iridium loaded on activated carbon as a catalyst with a mass content of iridium , feed pressure and be the hydrogen of 50Kpa, at 30 ℃, hydrogenation reaction 5 hours, the mass ratio of methanol and aspartame is 8: 1; The mass ratio of catalyst and aspartame is 0.05: 1;
[0027] (2) Remove the catalyst by filtration, concentrate the filtrate to remove methanol, and purify by recrystallization to obtain neotame with a yield of 90% and a purity greater than 99%.
Embodiment 2
[0029] A method for synthesizing neotame, comprising the steps of:
[0030] (1) In methanol, 3,3-dimethylbutyraldehyde and aspartame with a molar ratio of 1.01:1 are under the catalysis of the iridium that is loaded on activated carbon as a catalyst with a mass content of iridium of 10%. , feed pressure and be the hydrogen of 1Kpa, at 30 ℃, hydrogenation reaction 15 hours, the mass ratio of methanol and aspartame is 8: 1; The mass ratio of catalyst and aspartame is 0.01: 1;
[0031] (2) Remove the catalyst by filtration, concentrate the filtrate to remove methanol, and purify by recrystallization to obtain neotame with a yield of 86% and a purity greater than 99%.
Embodiment 3
[0033] A method for synthesizing neotame, comprising the steps of:
[0034] (1) in ethanol, be that 1.05: 1 3,3-dimethylbutyraldehyde and aspartame in the mass content of iridium are 15% under the catalysis of the iridium that is loaded on the activated carbon as catalyst , feed pressure and be the hydrogen of 150Kpa, at 20 ℃, hydrogenation reaction 30 hours, the mass ratio of ethanol and aspartame is 12: 1; The mass ratio of catalyst and aspartame is 0.5: 1;
[0035] (2) Remove the catalyst by filtration, concentrate the filtrate to remove ethanol, and purify by recrystallization to obtain neotame with a yield of 85% and a purity greater than 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com