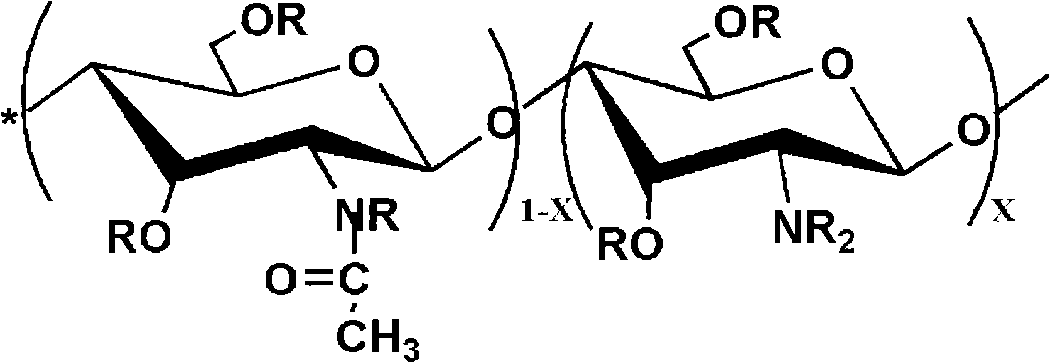
Preparation method of organic soluble photosensitive chitosan derivative
A technology of chitosan derivatives and organic dissolution, which is applied in the field of preparation of organic soluble photosensitive chitosan derivatives, and can solve the problems of reducing the application value of chitosan derivatives, many by-products of the reaction, and high energy consumption, etc. problems, to achieve good UV absorption capacity, save raw materials and costs, and increase the effect of application range
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
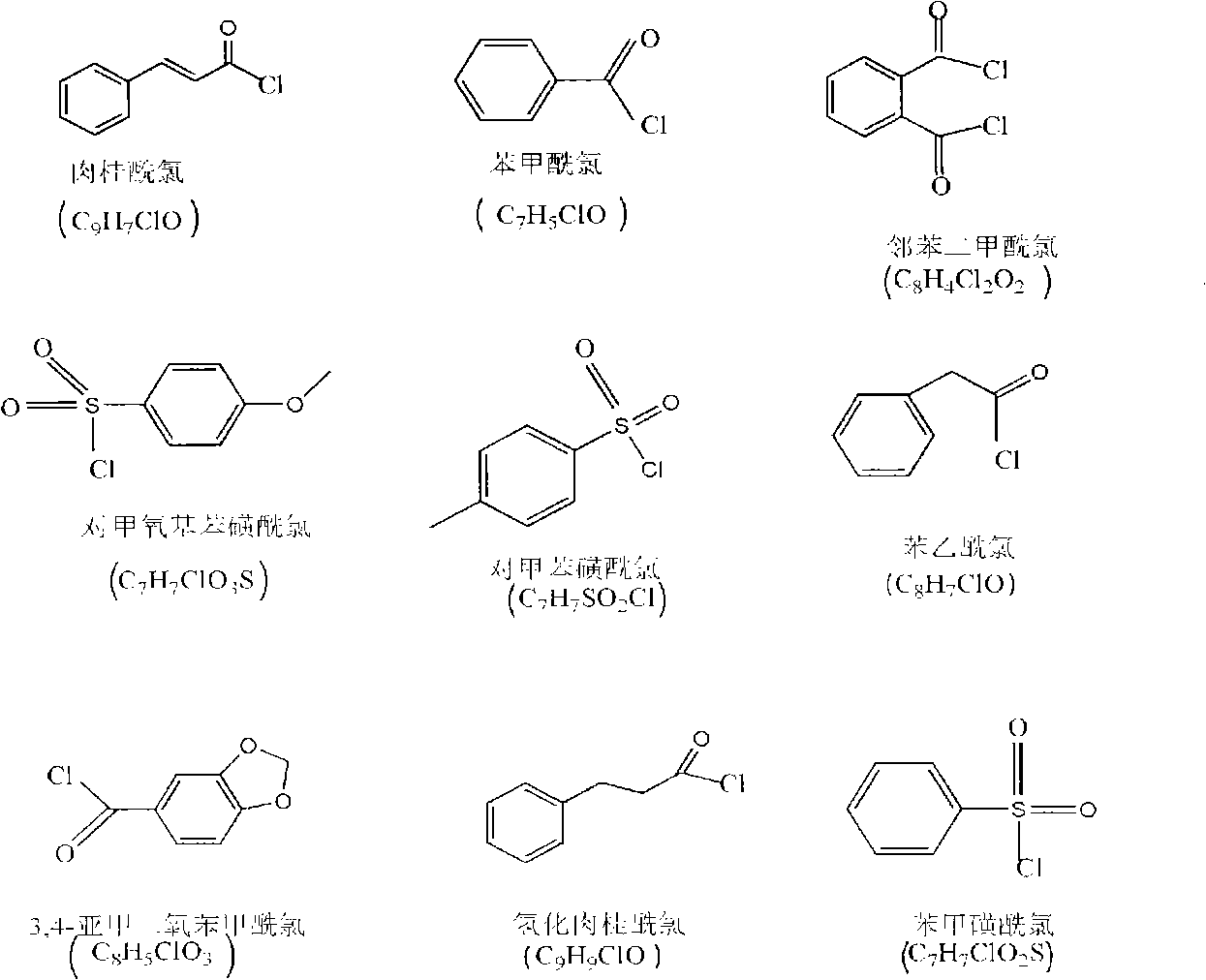
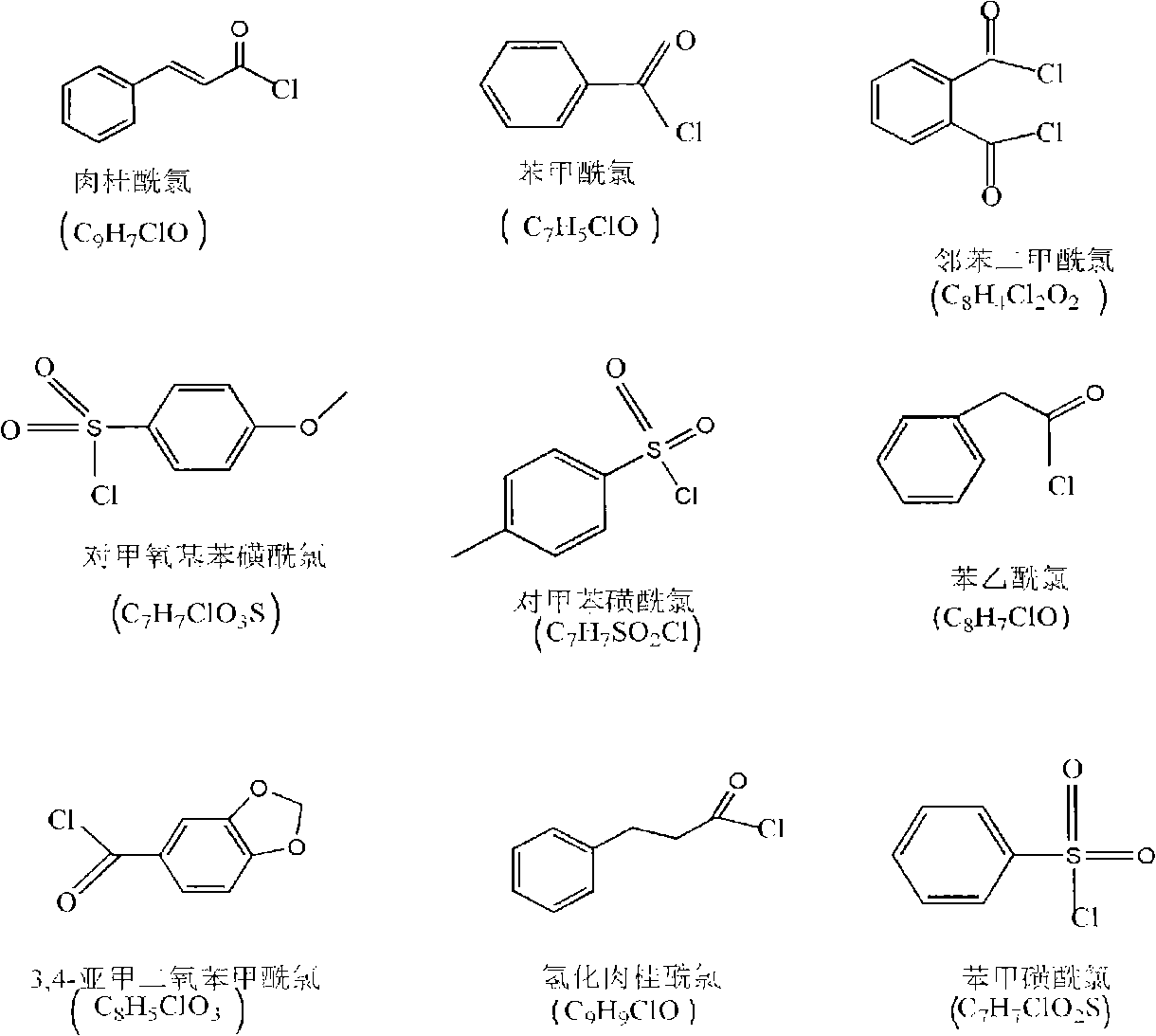
[0028] Add 2 g of chitosan (degree of deacetylation DP=80%, weight average molecular weight Mw=500,000) into 70 ml of triethylamine, stir at room temperature to make chitosan evenly dispersed in triethylamine; add cinnamoyl chloride In the carbon tetrachloride solution, the carbon tetrachloride solution of cinnamoyl chloride is prepared, wherein the addition amount of photosensitive acid chloride monomers is 0.5 times the mole number of amino groups, imino groups and hydroxyl groups on chitosan; The monomer-like carbon tetrachloride solution was slowly added dropwise to the chitosan solution with constant stirring; after all the dropwise additions were completed, the reaction was continued for 6 h. After the reaction was completed, suction filtration was performed to obtain a powdery solid, which was then used 8wt% sodium bicarbonate solution was washed three times with water, the washed product was filtered, and vacuum dried to obtain a photosensitive chitosan derivative with ...
Embodiment 2
[0034]Add 2 g of chitosan (degree of deacetylation DP=70%, weight average molecular weight Mw=1,000,000) into 70 ml of triethylamine, stir at room temperature to make chitosan evenly dispersed in triethylamine; add benzoyl chloride Add it to the carbon tetrachloride solution to prepare a carbon tetrachloride solution of benzoyl chloride, wherein the amount of photosensitive acid chloride monomers added is twice the mole number of amino groups, imino groups and hydroxyl groups on chitosan; The carbon tetrachloride solution of the acyl chloride monomer was slowly added dropwise to the chitosan solution, and kept stirring; after all the dropwise additions were completed, the reaction was continued for 4 hours. After the reaction was completed, suction filtration was performed to obtain a powdery solid. Then washed with 8wt% sodium bicarbonate for 3 times, the washed product was filtered and dried in vacuum to obtain a photosensitive chitosan derivative with a substitution degree o...
Embodiment 3
[0040] Add 2g of chitosan (degree of deacetylation DP=50%, weight average molecular weight Mw=100,000) into 70ml of triethylamine, stir at room temperature to make chitosan evenly dispersed in triethylamine; The benzenesulfonyl chloride is added to the carbon tetrachloride solution to prepare a carbon tetrachloride solution of p-methoxybenzenesulfonyl chloride, wherein the addition amount of the photosensitive acid chloride monomer is the amino group, imino group and hydroxyl group on the chitosan 3 times the number of moles; slowly drop the carbon tetrachloride solution of the photosensitive acid chloride monomer into the chitosan solution, and keep stirring; after all the dropwise additions are completed, continue the reaction for 6 hours, and after the reaction is completed, carry out Suction filtration to obtain a powdery solid, then washed three times with 8wt% sodium bicarbonate water, filtered the washed product, and vacuum dried to obtain a photosensitive chitosan deriv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com