Polypeptide with inhibiting virus mixed infection activity
A virus and active ingredient technology, applied in the direction of antiviral agents, medical preparations containing active ingredients, peptides, etc., can solve the problems of complicated epidemic situation, difficult diagnosis and prevention of livestock and poultry diseases, etc., and achieve good stability and inhibitory ability strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
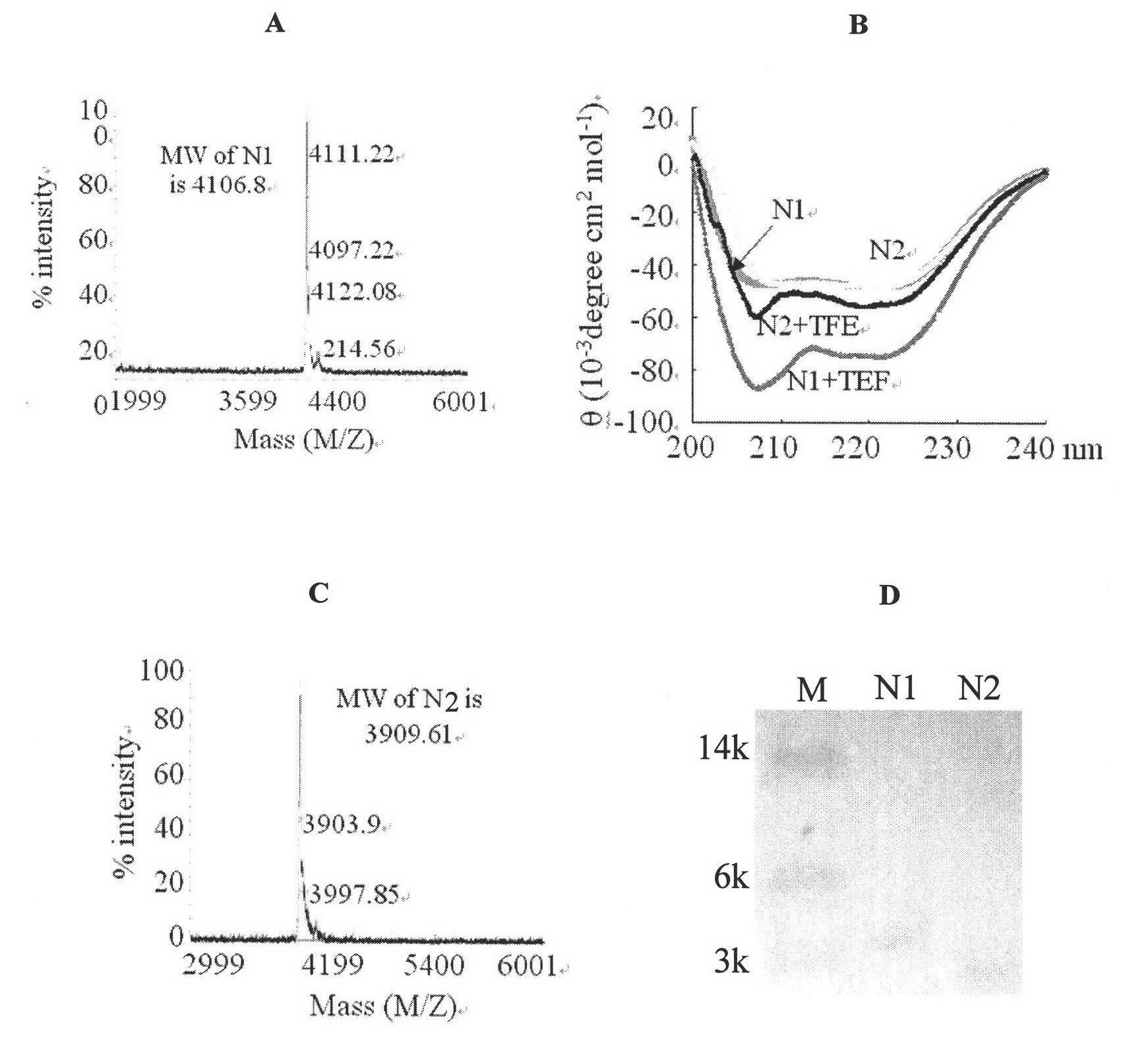
[0073] Example 1. Preparation and functional testing of novel polypeptides
[0074] 1. Preparation of new peptides
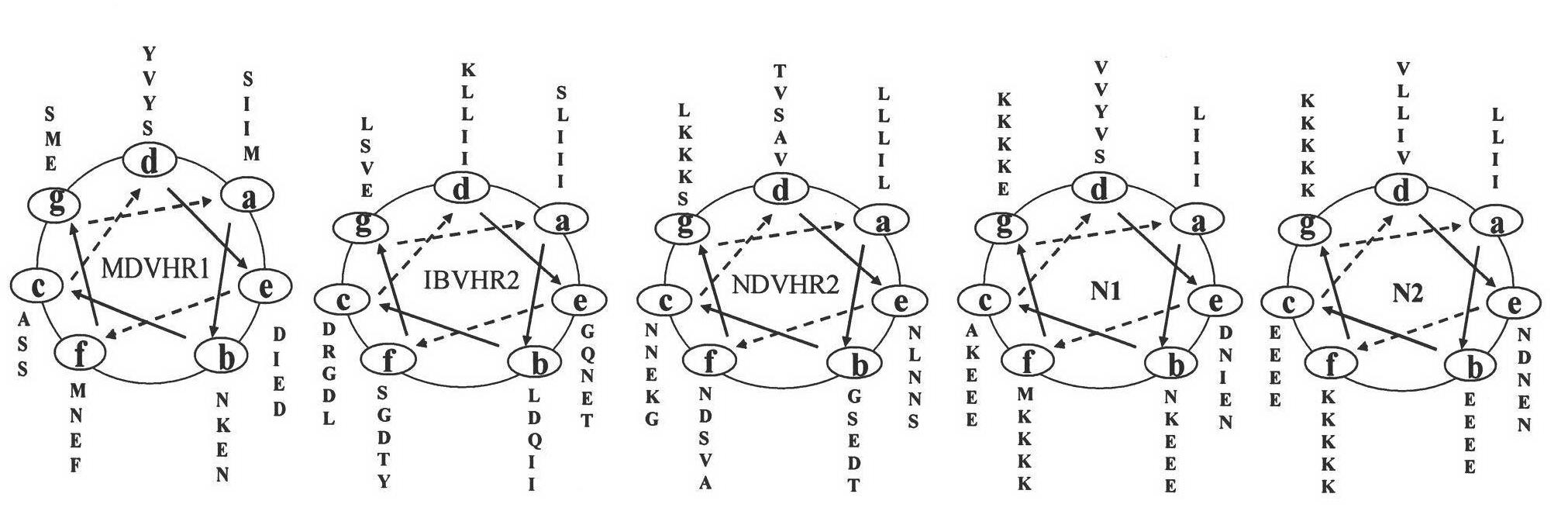
[0075] Gene redesign and splicing were carried out on the basis of the sequence comparison of the HR regions of the two viruses (NDV and IBV), that is, according to the inherent characteristics of the HR region—the a site is highly conserved (mostly leucine I / isoleucine L), the non-important helix-forming unit is replaced by lys / glutamic acid without affecting the structure-in short, the designed HR polypeptide will only need to consider the amino acid properties of the d and e sites in the HR regions of two different viruses different( figure 1 ).
[0076] PGEX-6p-1 vector (purchased from Amersia Company, catalog number is P0048); BL21(DE3) bacteria (purchased from Beijing Kangwei Century Company, catalog number is CW0809).
[0077] 1. The amino acid sequence of the N1 polypeptide is: NASDMEIKKVNKKIEEYIKKIEEVEKKLEEVNKK (sequence 1 in the sequence listing). ...
Embodiment 2
[0182] Embodiment 2, preparation and functional detection of control polypeptide
[0183] 1. Antiviral activity of HR2 polypeptide of IBV, HR2 polypeptide of NDV and HR2 polypeptide of MDV
[0184] (1) Preparation of each polypeptide
[0185] 1. Gene cloning, protein expression and purification: Respectively digest and recover the following PCR products and clone them into the PGEX-6p-1 vector (purchased from Amersia Company, catalog number P0048), and transform the positive clone into BL21 (DE3 ) bacteria (purchased from Beijing Kangwei Century Company, the product catalog number is CW0809) for protein expression, the supernatant was obtained by centrifugation after ultrasonic cracking of the expressed bacteria, and the supernatant was passed through a Glutathione-Sepharose 4B affinity chromatography column equilibrated with PBS ( (purchased from Oriental Science and Technology Company), and then wash the affinity column with PBS for at least 10 column volumes, followed by a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com