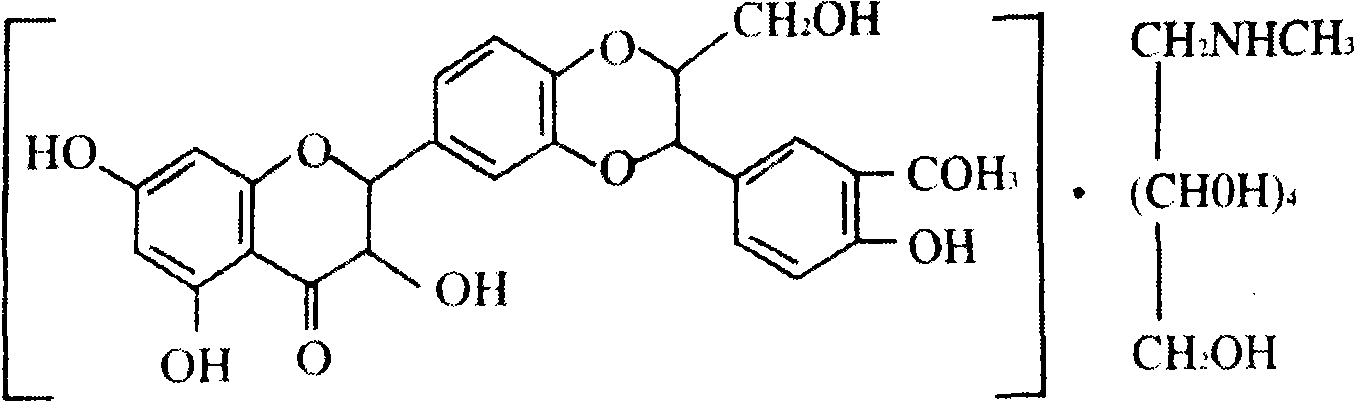
Production method of silybin meglumine tablets
A technology of silibinin and meglumine tablets, which is applied in the direction of pharmaceutical formulas, medical preparations containing no active ingredients, medical preparations containing active ingredients, etc., can solve the problems of high cost investment and brittle packaging materials, etc. Achieve the effect of basic quality indicators, stable quality indicators, and accelerated detoxification ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Embodiment 1: the present invention is a kind of production method of silibinin meglumine tablet, and this method is: respectively take by weighing 48.85g of silybin meglumine, lactose 91.59g, carboxymethyl starch sodium 11.29g, dioxidized Silicon 10.10g, pregelatinized starch 38.67g, magnesium stearate 3.05g, the mass content of each crude drug is obtained by calculation: silibinin meglumine 24%, lactose 45%, sodium starch glycolate 5.55% %, silicon dioxide 5%, pregelatinized starch 19%, magnesium stearate 1.5%; then the above-mentioned silibinin meglumine, lactose, sodium starch glycolate, silicon dioxide, pregelatinized The starch is made into a soft material with ethanol with a volume content of 80%, followed by 12-mesh granulation, 60°C drying, 14-mesh granulation, adding magnesium stearate, mixing, tableting, coating, and packaging. The silybin meglumine tablet adopts polyvinyl chloride (PVC) as packaging material during wherein packaging.
[0022] Existing produ...
Embodiment 2
[0025] Embodiment 2: the present invention is a production method of silibinin meglumine tablets, the method is: respectively weigh 52.92g of silybin meglumine, 85.95g of lactose, 18.30g of sodium starch glycolate, and Silicon 18.30g, pregelatinized starch 27.00g, magnesium stearate 1.08g; through calculation, the mass content of each raw material medicine is respectively: silibinin meglumine 26%, lactose 42.2%, carboxymethyl starch sodium 9 %, silicon dioxide 9%, pregelatinized starch 13.3%, magnesium stearate 0.5%. Then the above-mentioned silibinin meglumine, lactose, sodium starch glycolate, silicon dioxide, and pregelatinized starch that were weighed above are made into a soft material with a volume content of 95% ethanol, and then successively carry out 16 mesh granulation, 80 ℃ drying, granulation at 14 meshes, adding magnesium stearate and mixing evenly, tableting, coating, and packaging to obtain the silybin meglumine tablets, wherein polyvinyl chloride (PVC) is used ...
Embodiment 3
[0026] Embodiment 3: the present invention is a kind of production method of silibinin meglumine tablet, and this method is: take by weighing 40.71g of silybin meglumine, lactose 81.42g, carboxymethyl starch sodium 20.35g, dioxide 20.35g of silicon, 38.67g of pregelatinized starch, and 2.05g of magnesium stearate; the mass content of each bulk drug is obtained by calculation: 20% of silibinin meglumine, 40% of lactose, and 10% of sodium starch glycolate %, silicon dioxide 10%, pregelatinized starch 19%, magnesium stearate 1.0%. Then the above-mentioned silibinin meglumine, lactose, sodium starch glycolate, silicon dioxide, and pregelatinized starch that were weighed above are made into a soft material with a volume content of 95% ethanol, and then successively carry out 14 mesh granulation, 50 ℃ drying, granulation at 16 mesh, adding magnesium stearate and mixing, tableting, coating, and packaging to obtain the silybin meglumine tablets, wherein polyvinyl chloride (PVC) is use...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com