LC-MS/MS analysis method for detecting morphinane alkaloid in whole blood and urine
A technology of LC-MS and analytical methods, applied in analytical materials, measuring devices, material separation, etc., can solve the problems of inability to meet the needs of case inspection, large polarity, difficulty in extraction and detection, etc., to simplify experimental steps and save money. Analyzing the effect of time
Inactive Publication Date: 2010-08-25
上海市公安局刑事侦查总队
View PDF2 Cites 9 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Due to the strong water solubility and high polarity of the 3-β-D glucuronide morphine, it is difficult to extract and detect, so there are few reports in the literature
Existing literature reports on the analysis of morphine alkaloids in biological samples by GC-MS must undergo steps such as solvent extraction and derivatization, and cannot detect the conjugates of morphine and glucuronic acid in biological samples- 3-beta-D glucuronide morphine, therefore does not meet the needs of the case test
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
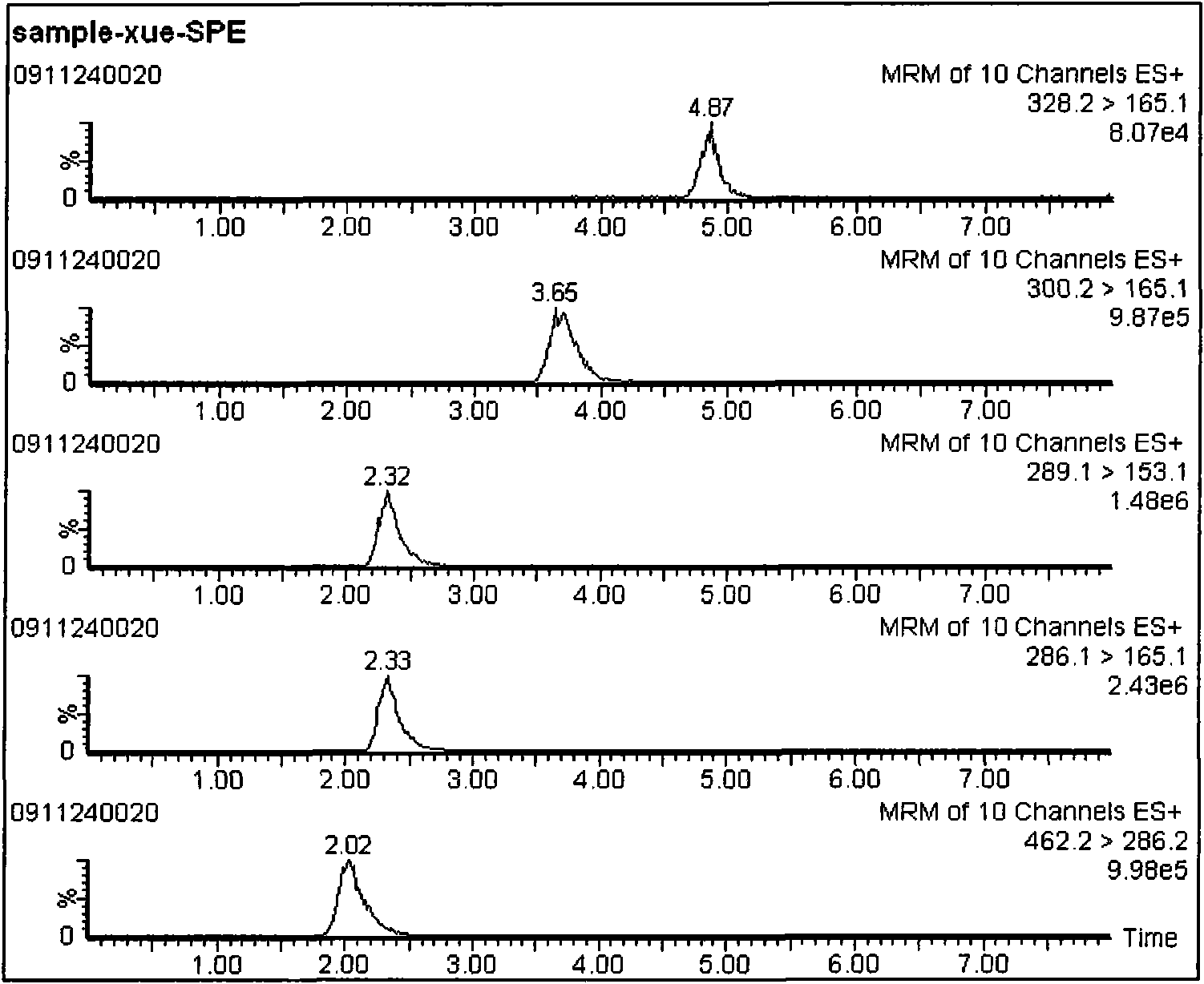
The invention discloses an LC-MS / MS analysis method for detecting morphinane alkaloid in whole blood and urine. The conditions of LC-MS and MS are separately as follows: 1) LC-MS chromatographic conditions: the mobile phase is composed of A and B, wherein A is an aqueous solution containing 2mmol / L of ammonium formate and 0.05% (v / v) of formic acid, B is acetonitrile containing 2mmol / L of ammonium formate and 0.05% (v / v) of formic acid, the volume ratio of A to B is 90:10; the column temperature is 45 DEG C; the flow rate is 0.2mL / min; the sampling volume is 10mu L; and 2) MS conditions: the detection mode adopts multiple reaction monitoring (MRM); the scanning mode adopts positive ion scanning; the electrospray voltage is 3200V; the atomization air flow rate is N2 600L / hr; the cone gas glow is N2 50L / hr; the ion source temperature is 105 DEG C; the collision gas is argon; and the internal standard adopts morphine-d3. The morphinane alkaloid contains morphine-3-beta-D-glucuronide, morphine, O6-monoacetylmorphine and codeine. The analysis method of the invention adopts solid phase extraction to purify the sample, thus the qualitative and quantitative measurements become more accurate; by adopting different elution and elution conditions, the obtained rate of recovery can be the optimal; and after the sample is added, methanol is used for further elution, thus simplifying the experimental procedures and reducing the analysis time.
Description
LC-MS / MS method for detecting morphine alkaloids in whole blood and urine technical field The invention relates to an LC-MS / MS analysis method for detecting morphine alkaloids in whole blood and urine, in particular to a method for detecting 3-beta-D glucuronide morphine, morphine and O6-monoacetyl in whole blood and urine LC-MS / MS method for the analysis of morphine and codeine. Background technique As the international drug tide continues to spread, foreign drugs are increasingly invading our country, and more and more people are taking or injecting heroin. Therefore, the requirements for the analysis and detection of morphine alkaloids in the human body are also getting higher and higher. In accordance with the Ministry of Public Security's "Definition of Drug Addiction Standards for Persons Who Take and Inject Drugs", all drug users who are seized and injected should undergo a forensic appraisal of drug addiction and identification of illegal drug use. Among them, the...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): G01N30/02G01N30/72G01N30/06
Inventor 张玉荣梁晨叶海英汪蓉
Owner 上海市公安局刑事侦查总队
Features
- Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com