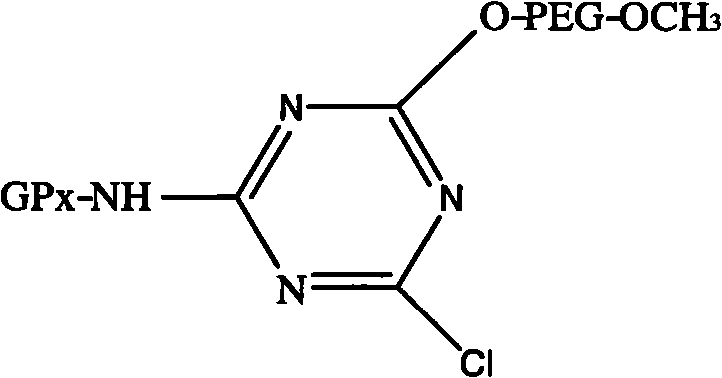
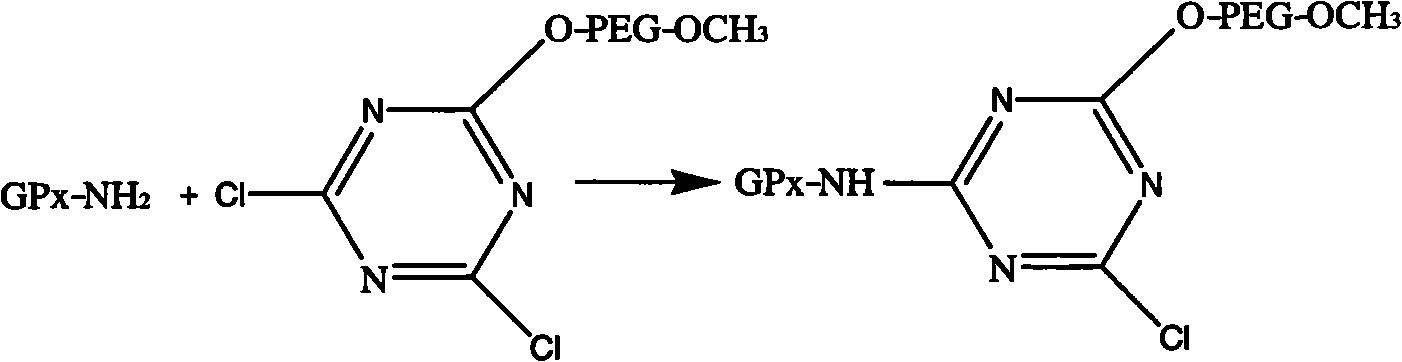
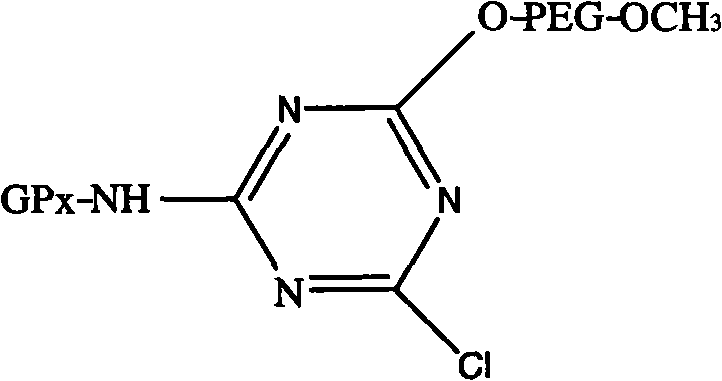
Modified glutathione peroxidase and preparation method thereof
A technology of glutathione peroxidase and glutathione peroxide, applied in the biological field, can solve the problems of easy oxidase, limited practicability, short half-life, etc., achieve high modification enzyme activity, eliminate enzyme protein The effect of antigen-antibody reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1. Activation of mPEG 1 The acquisition of:
[0024] Take 5.5 g of cyanuric chloride recrystallized twice (recrystallized twice with anhydrous benzene first) and dissolve it in 400 mL of anhydrous benzene containing 10 g of anhydrous sodium carbonate, add 50 g of mPEG-5000, stir overnight at room temperature, filter , take about 400mL of filtrate and stir and slowly add 600mL of diethyl ether, continue to stir for 20min after the white product precipitates, filter with suction, and dissolve the precipitate with 400mL of anhydrous benzene. up to the absorption peak. Place the activated mPEG in a vacuum desiccator to dry it up to obtain a white powder, that is, to obtain activated mPEG 1 .
[0025] 2. Modification of glutathione peroxidase:
[0026] The reaction temperature is 15°C, the pH is 9.0, the reactant ratio is 1 mg of enzyme, 0.08 g of modifier is added for reaction, after 45 min, GSH is added as the terminator of GPx modification reaction to end the reaction...
Embodiment 2
[0028] 1. Activation of mPEG 1 Acquisition: as in Example 1
[0029] 2. Modification of glutathione peroxidase:
[0030] The reaction temperature is 36°C, pH 7.0, and the reactant ratio is 1 mg of enzyme and 0.04 g of modifier for reaction. After 30 minutes, GSH is added as the terminator of GPx modification reaction to end the reaction, and the modified glutathione is obtained after ultrafiltration to remove the terminator. Glycerin peroxidase.
Embodiment 3
[0032] 1. Activation of mPEG 1 Acquisition: as in Example 1
[0033] 2. Modification of glutathione peroxidase:
[0034] The reaction temperature is 5°C, the pH is 10.0, and the reactant ratio is 1 mg of enzyme and 0.02 g of modifier for reaction. After 60 minutes, GSH is added as the terminator of GPx modification reaction to end the reaction, and the modified glutathione is obtained after ultrafiltration to remove the terminator. Glycerin peroxidase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com