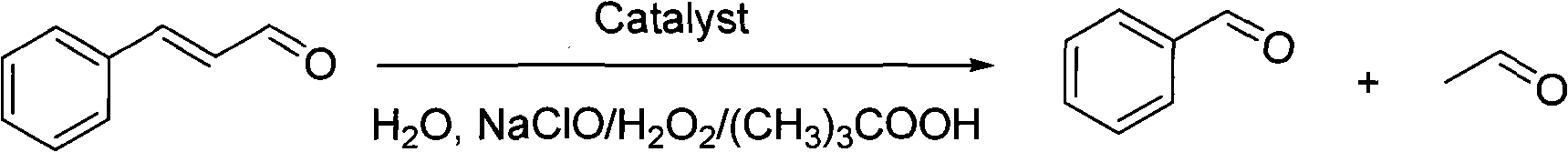Method for preparing benzaldehyde by using cyclodextrin polymer to catalyze oxidation of cinnamic aldehyde or cinnamon oil
A technology of cyclodextrin polymer and benzaldehyde, which is applied in the oxidation preparation of carbonyl compounds, organic chemistry, etc., can solve the problems of low ozone utilization rate, low benzaldehyde naturalness, high equipment requirements, etc., and achieve easy control and low cost , The effect of simple reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
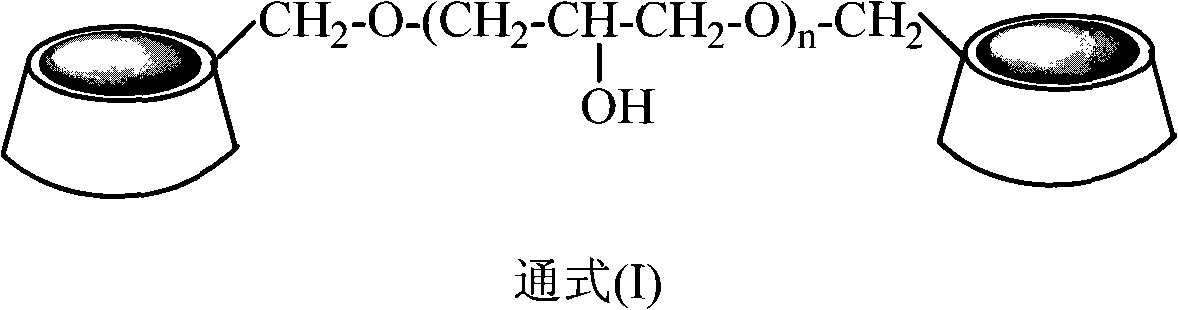
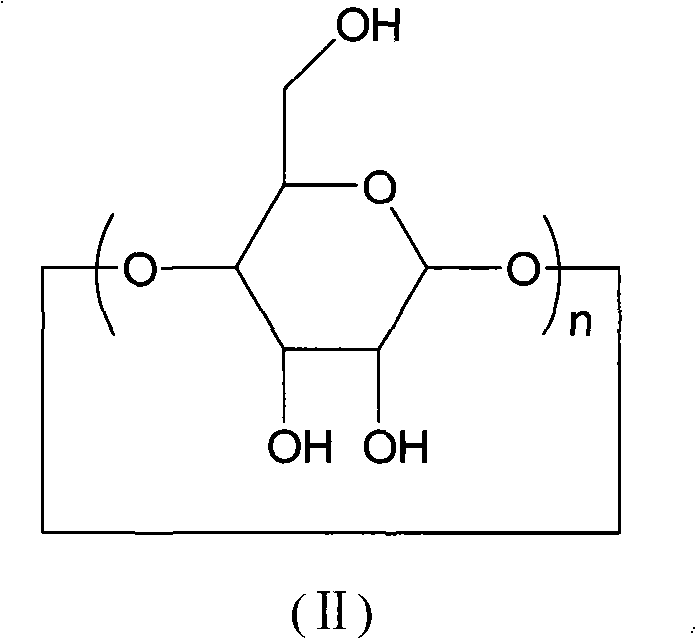
[0026] Add 3.362g (1mmol) of β-cyclodextrin polymer (n=15 in general formula (I)) and 25ml of deionized water to a 100ml flask respectively, and stir at 30°C until the β-cyclodextrin polymer is uniformly dispersed , then add 1mmol cinnamaldehyde and 6ml30% hydrogen peroxide solution successively, after the reaction, extract with 50ml ethyl acetate, the ethyl acetate phase of gained can obtain the yield of 47% benzaldehyde by concentrating under reduced pressure to remove ethyl acetate .
Embodiment 2
[0028] Add 3.258g (1mmol) of α-cyclodextrin polymer (n=18 in general formula (I)) and 25ml of deionized water to a 100ml flask respectively, and stir at 70°C until the α-cyclodextrin polymer is uniformly dispersed , then add 1mmol cinnamaldehyde and 6ml 30% hydrogen peroxide solution successively, after the reaction finishes, extract with 50ml ethyl acetate, the ethyl acetate phase of gained can obtain the benzene that yield is 78% through concentrating under reduced pressure and removing ethyl acetate. formaldehyde.
Embodiment 3
[0030] Add 4.130g (1mmol) of γ-cyclodextrin polymer (n=21 in general formula (I)) and 25ml of deionized water to a 100ml flask respectively, and stir at 90°C until the γ-cyclodextrin polymer is evenly dispersed , then add 1mmol cinnamaldehyde and 6ml 30% hydrogen peroxide solution successively, after the reaction finishes, extract with 50ml butyl acetate, the butyl acetate phase of gained can obtain the benzene that yield is 59% through concentrating under reduced pressure and removing butyl acetate formaldehyde.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com