Method for preparing calcium carbonate from yellow phosphorus furnace slag to co-produce industrial salt and silicon gel
A technology for yellow phosphorus slag and calcium carbonate is applied in the field of preparing calcium carbonate to co-produce industrial salt and silica gel, and achieves the effects of large market demand, low production cost and elimination of chemical waste residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
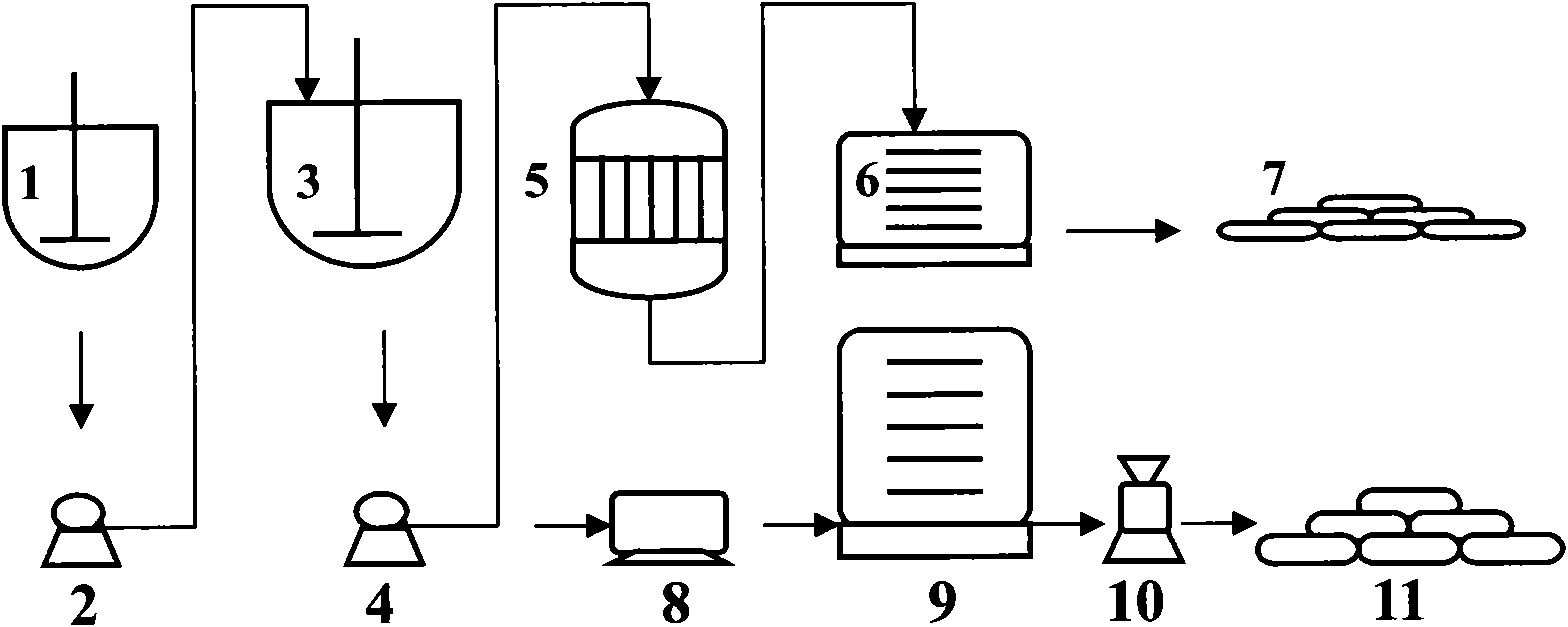
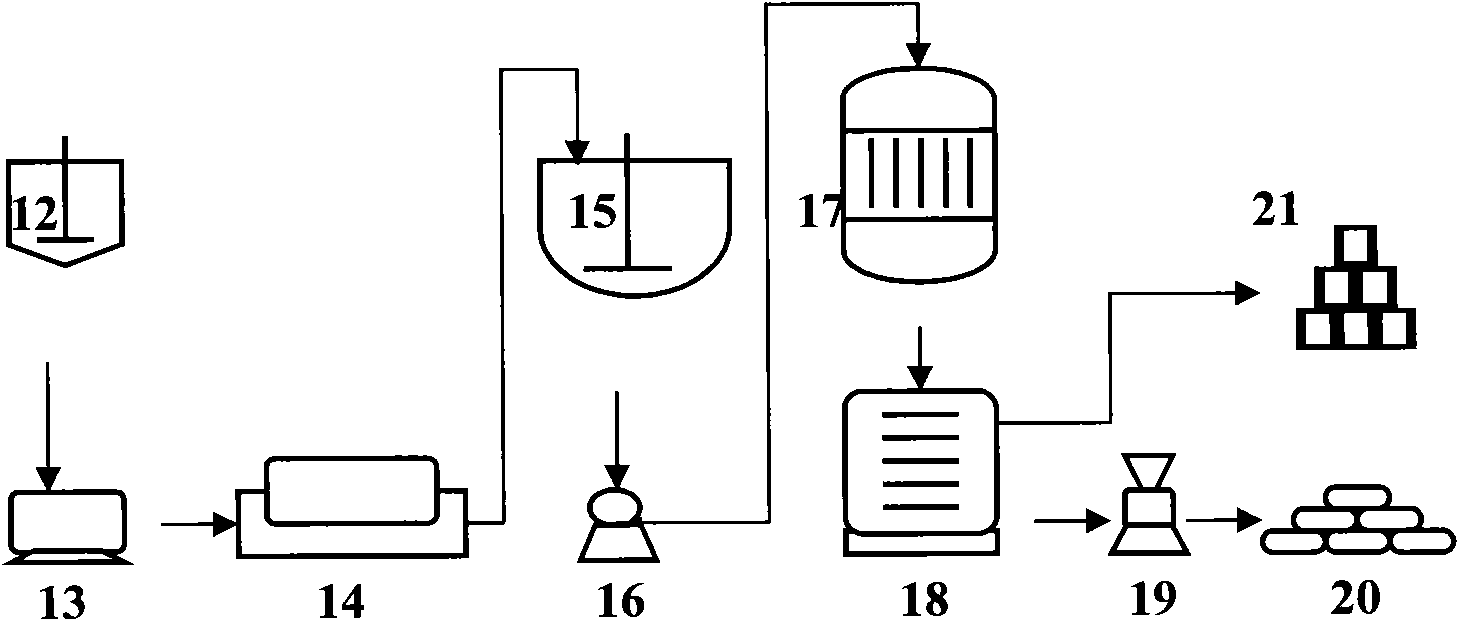
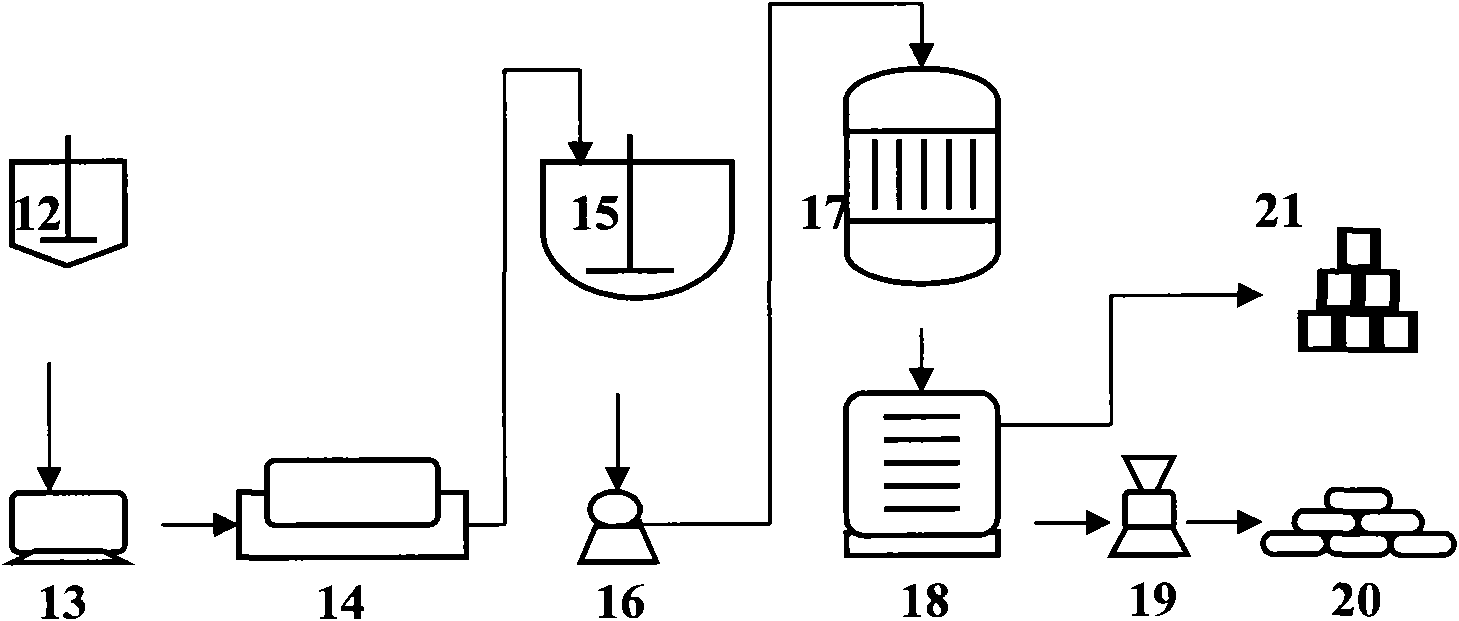
[0042]A method for preparing calcium carbonate co-production industrial salt and silica gel by using yellow phosphorus slag, including the purification and synthesis process of yellow phosphorus slag and the process of synthesizing silica gel;
[0043] The process of purifying and synthesizing the yellow phosphorus slag is as follows: 300 kg of pure calcium oxide in the converted yellow phosphorus slag and hydrochloric acid solution are placed in the purifier 1 to react according to the mass ratio of pure substances of 1:1.1, and then the reaction After the material obtains clarified 501.78kg calcium chloride solution and silica filter cake through the first suction filter 2 suction filtration;
[0044] Wherein, the clarified calcium chloride solution and sodium carbonate are put into the synthesizer 3 according to the mass ratio of pure substances of 1:0.76, and the chemical reaction is carried out under gentle stirring, and then the reacted substance is filtered through the s...
Embodiment 2
[0047] A method for preparing calcium carbonate co-production industrial salt and silica gel by using yellow phosphorus slag, including the purification and synthesis process of yellow phosphorus slag and the process of synthesizing silica gel;
[0048] The process of purifying and synthesizing the yellow phosphorus slag is as follows: 300 kg of pure calcium oxide in the converted yellow phosphorus slag and hydrochloric acid solution are placed in the purifier 1 to react according to the mass ratio of pure substances of 1:1.3, and then the reaction After the material obtains clarified 593.01kg calcium chloride solution and silica filter cake through first suction filter 2 suction filtration;
[0049] Wherein, the clarified calcium chloride solution and sodium carbonate are put into the synthesizer 3 according to the mass ratio of pure substances of 1:0.96, and the chemical reaction is carried out under gentle stirring, and then the reacted substance is filtered through the seco...
Embodiment 3
[0052] A method for preparing calcium carbonate co-production industrial salt and silica gel by using yellow phosphorus slag, including the purification and synthesis process of yellow phosphorus slag and the process of synthesizing silica gel;
[0053] The process of purifying and synthesizing the yellow phosphorus slag is as follows: 300 kg of pure calcium oxide in the converted yellow phosphorus slag and hydrochloric acid solution are placed in the purifier 1 to react according to the mass ratio of pure substances of 1:1.5, and then the reaction After the material obtains clarified 648.25kg calcium chloride solution and silica filter cake through first suction filter 2 suction filtration;
[0054] Wherein, the clarified calcium chloride solution and sodium carbonate are put into the synthesizer 3 according to the mass ratio of pure substance 1: 1.16, carry out the chemical reaction under gentle stirring, and then the reacted substance is filtered through the second suction f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com