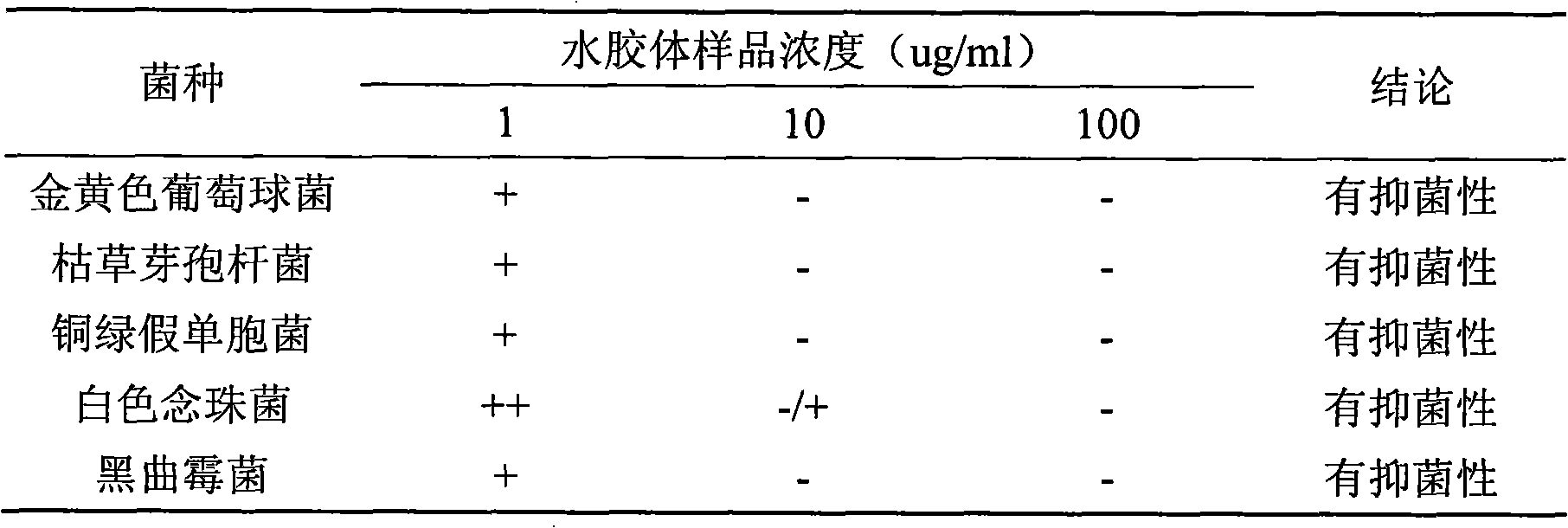
Bacteriostatic hydrocolloid dressing and preparation method thereof
A hydrocolloid and hydrophilic colloid technology, applied in the fields of biological materials and medical supplies, can solve the problems of not being able to give full play to the biological activity of chitosan, not improving the quality of wound healing, and single function, and achieving excellent ability to absorb exudate , Good healing environment, strong paste effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Take 1.5 kg of medical-grade ethylene propylene rubber, 1.0 kg of gelatin, 1 kg of mineral oil and 0.2 kg of phytic acid, mix them thoroughly with an internal mixer, then add 3.0 kg of sodium carboxymethyl cellulose, 1.0 kg of calcium alginate, 1.0 kg of viscose resin, 1.2 kg of O-carboxymethyl chitosan, and 0.1 kg of dry nano silver powder are mixed uniformly to form a blend. Among them, mineral oil and tackifying resin are both medical grade; O-carboxymethyl chitosan has a molecular weight of 100,000 to 500,000, a degree of deacetylation of 70%, a degree of carboxymethylation of 0.5 to 0.7, and a degree of substitution of the 6-hydroxyl 0.9; The particle size of the dry nano-silver powder is 100-1000nm; the molecular weight of sodium carboxymethyl cellulose is 20,000-50,000, and the degree of deacetylation is 70%. The above blend is uniformly coated on one side of a microporous polyurethane membrane with a pore size of 0.2μm-10μm and a thickness of 0.01mm-0.02mm to for...
Embodiment 2
[0027] Take 2.0 kg of medical grade ethylene propylene rubber, 1.0 kg of gelatin, 1 kg of mineral oil and 0.2 kg of phytic acid, mix them thoroughly with an internal mixer, then add 2.5 kg of sodium carboxymethyl cellulose, 1.0 kg of calcium alginate, 1.0 kg of viscose resin, 1.2 kg of O-carboxymethyl chitosan, and 0.1 kg of dry nano silver powder are mixed uniformly to form a blend. Among them, mineral oil and tackifying resin are both medical grade; O-carboxymethyl chitosan has a molecular weight of 500,000-1,000,000, a degree of deacetylation of 75%, a degree of carboxymethylation of 0.7-0.9, and a degree of substitution of the 6-hydroxyl It is 0.7; the molecular weight of sodium carboxymethyl cellulose is 80,000-150,000, and the degree of deacetylation is 75%; the particle size of the nano-silver dry powder is 1000-3000nm. The above blend is uniformly coated on one side of a microporous polyurethane membrane with a pore size of 0.2μm-10μm and a thickness of 0.01mm-0.02mm to...
Embodiment 3
[0030] Take 1.5kg of medical grade ethylene propylene rubber, 0.8kg of gelatin, 1kg of mineral oil and 0.2kg of phytic acid, mix them thoroughly with an internal mixer, then add 3.0kg of sodium carboxymethylcellulose, 1.2kg of calcium alginate, 1.0 kg of viscose resin, 1.2 kg of O-carboxymethyl chitosan, and 0.1 kg of dry nano silver powder are mixed uniformly to form a blend. Among them, mineral oil and tackifying resin are both medical grade; O-carboxymethyl chitosan has a molecular weight of 1,000,000-1,500,000, a degree of deacetylation of 80%, a degree of carboxymethylation of 0.9-1.2, and a degree of substitution of the 6-position hydroxyl group. It is 0.8; the molecular weight of sodium carboxymethyl cellulose is 200,000-300,000, and the degree of deacetylation is 85%; the particle size of nano-silver dry powder is 3000-6000 nm. The above blend is uniformly coated on one side of a microporous polyurethane membrane with a pore size of 0.2μm-10μm and a thickness of 0.01mm-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com