Bismaleimide containing 1,3,4-oxadiazole structure and preparation method thereof
A technology of bismaleimide and bismaleamic acid is applied in the field containing 1, which can solve the problems of poor processing characteristics and high melting point, and achieve the effects of increasing solubility, improving flexibility, and reducing melting point.
Inactive Publication Date: 2010-10-27
DALIAN UNIV OF TECH +1
View PDF2 Cites 8 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Its solubility has been significantly improved. It can be dissolved in chloroform, dichloromethane, N, N-dimethylformamide and other solvents, but the melting point is still high (266 ° C, 263 ° C), and the processing characteristics are also poor.
And not only containing high thermal oxidation resistance 1,3,4-oxadiazole heterocyclic ring but also containing bismaleimide and its preparation method of rotatable fatty ether linkage, there is no published patent or bibliographical report so far
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
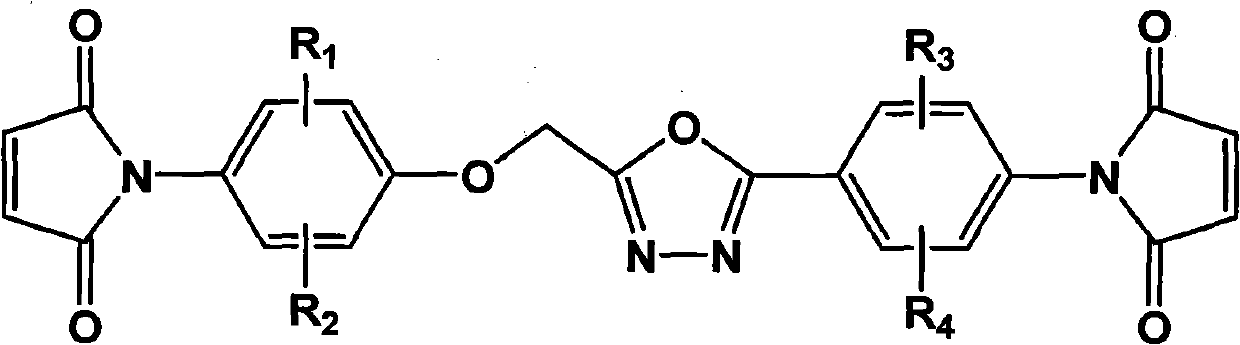
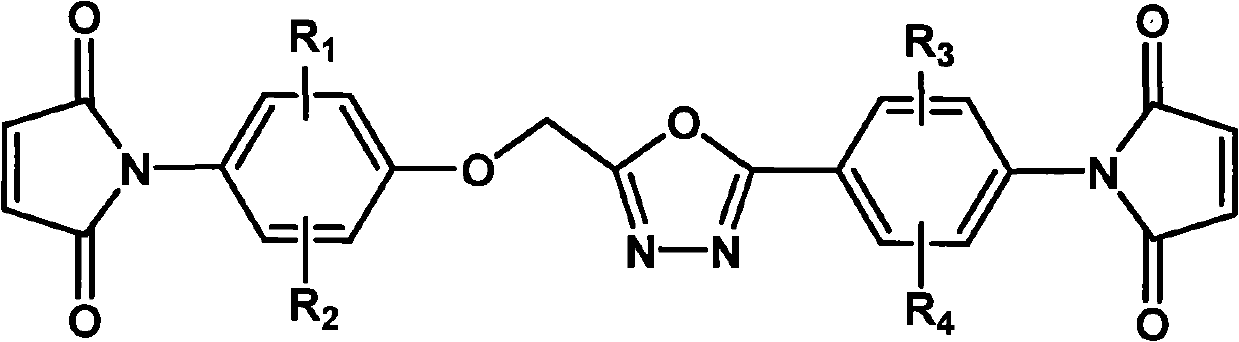
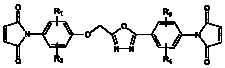
The invention relates to a bismaleimide containing a 1,3,4-oxadiazole structure and a preparation method thereof. In the structural formula of bismaleimide, substituents of R1 to R4 can be hydrogen atom, halogen atom, or same or different fat alkane of C1 to C20 and derivatives thereof, or same of different aromatic of C6 to C12 and derivatives thereof. The bismaleimide with a 1,3,4-oxadiazole structure is prepared by reacting diamine with a 1,3,4-oxadiazole structure with maleic anhydride to produce bismaleamic and performing imide cyclization reaction under the action of catalyst and dehydrating agent. The bismaleimide containing the1,3,4-oxadiazole structure has an asymmetric structure; and two reactive groups have different reaction activities in different chemical environments and can successively react at different curing temperatures. In addition, the cured product of the bismaleimide with a 1,3,4-oxadiazole structure has excellent heat resistance and is suitable for being used as a high-performance polymer composite matrix.
Description
technical field The invention belongs to the technical field of polymer materials, and in particular relates to a bismaleimide containing a 1,3,4-oxadiazole structure and a preparation method thereof. Background technique Bismaleimide is an important thermosetting polyimide with excellent properties of polyimide, such as: good thermal stability, electrical insulation properties, excellent chemical corrosion resistance, radiation resistance, heat and humidity resistance and mechanical properties, etc. At the same time, bismaleimide is a small molecule, so it has the easy processing performance similar to epoxy resin, and the molding process is flexible. The double bonds at both ends of the molecule have strong electrophilicity due to the strong electron-withdrawing effect of two carbonyl groups. It can undergo nucleophilic addition reactions with nucleophilic groups such as diamines and diphenols, and can also react with allyl compounds. Copolymerization of donating unsatur...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D413/14
Inventor 陈平熊需海马克明王柏臣于祺
Owner DALIAN UNIV OF TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com