Primer group for detecting Bordetella pertussis, detection test kit and detection method
A technology for detection kits and detection methods, applied in biochemical equipment and methods, recombinant DNA technology, microbiological determination/inspection, etc., can solve the problem that children cannot produce normal immune responses to pathogens, are not suitable for early diagnosis, early diagnosis Difficulties and other problems, to achieve the effect of saving testing costs, reducing pollution opportunities, and easy and fast operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 primer set
[0023] By comparing and analyzing B. pertussis IS481 gene and PT gene in Genbank database, design amplification primer pair, IS481 gene primer pair sequence is the nucleotide sequence shown in SEQ ID NO: 1 and SEQ ID NO: 2; PT gene A pair of amplification primers, the sequences of the primer pair are SEQ ID NO: 3 and SEQ ID NO: 4.
Embodiment 2 100
[0024] Example 2 Preparation of Bacillus pertussis double PCR detection kit
[0025] The composition of the kit:
[0026] IS481 gene amplification primers:
[0027] P15'-gatgtcggtggcgctgtt-3' (SEQ ID NO: 1),
[0028] P25'-gagaaactggaaatcgcca-3' (SEQ ID NO: 2);
[0029] PT gene amplification primers:
[0030] P15'-gcatgcgtgcagattcgtcgt-3' (SEQ ID NO: 3),
[0031] P25'-aacgcagagggggaagacg-3' (SEQ ID NO: 4).
[0032] Negative control: high pressure double distilled water
[0033] Positive control: pertussis standard strain DNA (or a plasmid that connects two fragments of IS481 gene and PT gene)
[0034] 10x buffer
[0035] MgCl 2
[0036] dNTP Mixture
[0037] Taq enzyme (can be selected from TaKara's rTaq or Ex Taq or other Taq enzymes with excellent performance).
[0038] The kit described in this implementation, under the premise of not affecting the effect of PCR amplification, can prepare buffers of different multiples according to needs; or mix buffer, MgCl 2 An...
Embodiment 3
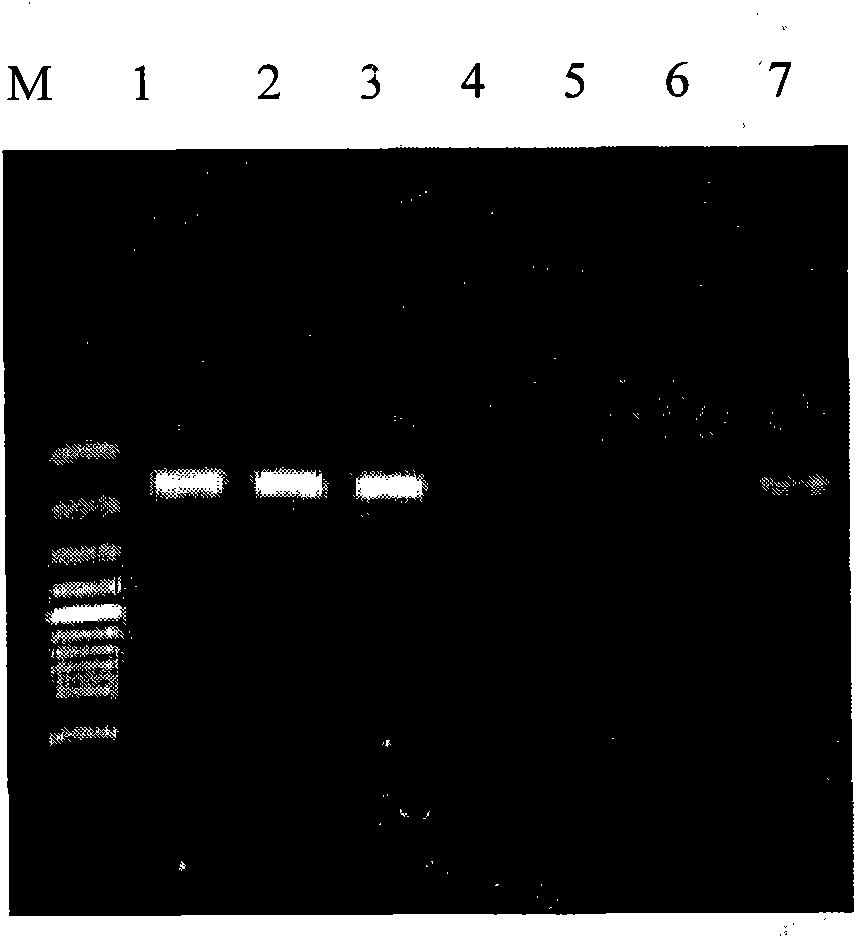
[0039] Embodiment 3 Double PCR amplifies IS481 gene and PT gene simultaneously
[0040] The test bacteria is the standard strain of pertussis, sourced from China Institute of Pharmaceutical and Biological Products, number (58001).
[0041] 1. Sample DNA (template) extraction:
[0042] Use Beijing Tiangen Bioengineering Company's Bacterial Genomic DNA Extraction Kit to extract sample DNA, DNA OD 260 / OD 280 In the range of 1.6-2.0, the concentration is 10-100ng / μl.
[0043] 2. Reaction system:
[0044] 10*buffer (buffer solution) 2.5ul
[0045] MgCl 2 1.5mM
[0046] dNTP Mixture (2.5mM each) 2.5ul
[0047] Upstream and downstream primers (10pmol / ul) 0.5ul
[0048] Template 5ul
[0049] Ex Taq (TaKara) 0.125ul
[0050] Add high pressure double distilled water to 25ul
[0051] 3. Carry out double PCR amplification reaction:
[0052] (1) Prepare a 20 μl reaction system using TaKaRa Ex Taq from Dalian Bao Biological Engineering Co., Ltd., and finally ad...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com