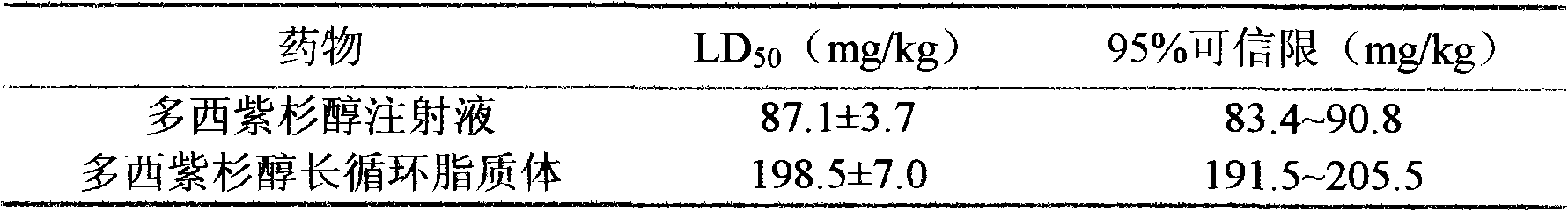Docetaxel long-circulating liposome and preparation method thereof
A long-circulating liposome and docetaxel technology, which is applied in the directions of liposome delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve problems such as systemic toxicity, damage, and clinical application limitations, and achieve increased curative effect. , avoid phagocytosis, increase the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Dissolve 1.0 mg of docetaxel, 100.0 mg of soybean lecithin, and 8.0 mg of cholesterol in 10 ml of ethanol to obtain solution A.
[0024] Film A was formed into a film by rotary evaporation in a water bath at 40°C to obtain film B.
[0025] Dissolve 200 mg of poloxamer in 5 ml of phosphate buffer at pH=6.8 to obtain solution C.
[0026] Add solution C to membrane B, refrigerate at below 4°C for 30 minutes to fully hydrate the membrane, and then vortex for 15 minutes to prepare the product.
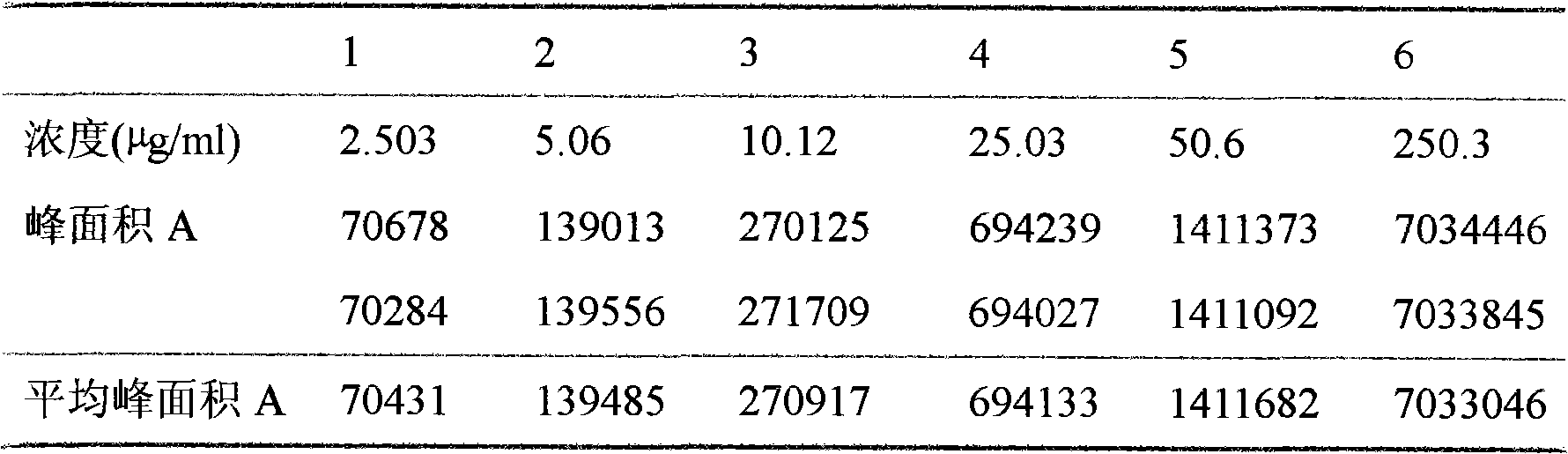
[0027] Apply Sephadex column to separate liposome and unencapsulated free drug, add methanol to dissolve, take methanol solution and apply high performance liquid chromatography to measure peak area, substitute peak area into regression equation to find concentration, calculate encapsulation The sealing rate is 90.3%.
[0028] Take about 10.0mg of the raw material drug, weigh it accurately, put it in a 10ml measuring bottle, add methanol to dissolve it ultrasonically, let it cool d...
Embodiment 2
[0033] Dissolve 4 mg of docetaxel, 80 mg of phosphatidylcholine, and 10 mg of cholesterol in 10 ml of ethanol and chloroform mixture (1:1, volume ratio) to obtain solution A.
[0034] Film A was formed into a film by rotary evaporation in a water bath at 40°C to obtain film B.
[0035] Dissolve 200 mg of poloxamer in 5 ml of pH=9 phosphate buffer to obtain solution C.
[0036] Add solution C to membrane B, refrigerate below -4°C for 30 minutes to fully hydrate the membrane, and then vortex and mix for 15 minutes to prepare the product.
Embodiment 3
[0038] By tail vein administration of embodiment 1 and docetaxel injection, measure two kinds of dosage forms median lethal dose (LD 50 ), using the modified Cole’s method to calculate, the results are as follows: the acute toxicity of docetaxel long-circulation liposome is significantly less than that of docetaxel injection, LD 50 The dose is more than twice that of docetaxel injection, indicating that the prepared long-circulating liposome of docetaxel can reduce the toxicity of the drug. The results are shown in the table below:
[0039] Docetaxel Injection and Docetaxel Long Circulation Liposome LD 50 (x±s)
[0040]
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com