Eosinophil analogue, preparation method thereof and whole blood quality control substance
A technology for eosinophils and neutrophils, applied in the field of whole blood quality control substances, can solve the problems of difficult quality control of eosinophils, low content of eosinophils, damage of white blood cell simulants, etc., to achieve convenient The effect of large-scale production, low production cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
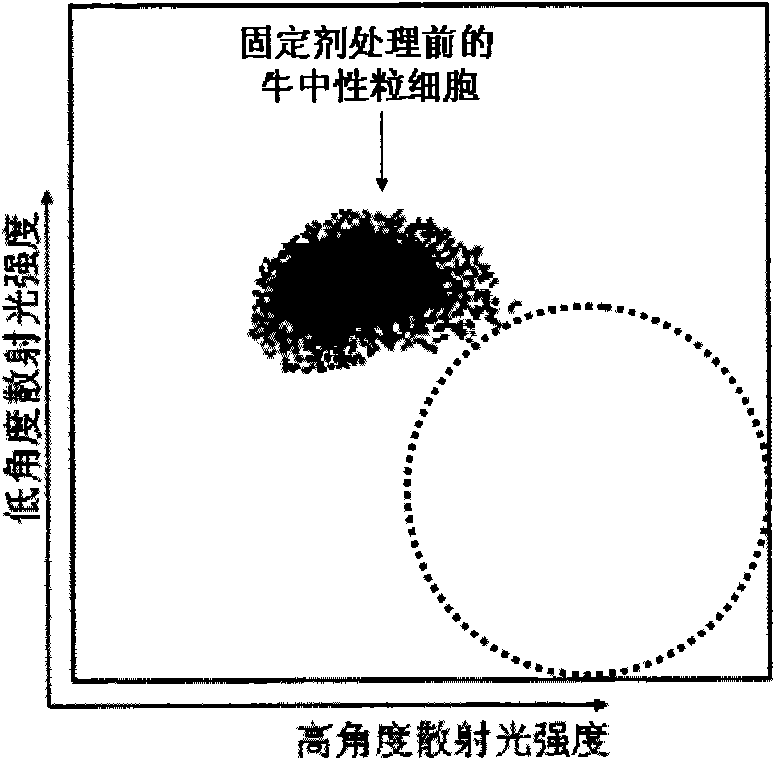
[0055] 1. Take 100ml of fresh anticoagulated bovine blood, containing 2g / L of anticoagulant;
[0056] 2. Mix 100ml of anticoagulated bovine blood with 400ml of hemolytic agent, stir for 10 minutes, centrifuge at 2000g for 5 minutes, and harvest white blood cells;
[0057] 3. Centrifuge and wash white blood cells with PBS buffer once or twice;
[0058] 4. Take the white blood cells in step 3 and adjust the white blood cell count to 50×10 with PBS 9 pieces / L, centrifugal force 200g, centrifuge for 10 minutes, harvest the precipitate;
[0059] 5. Get the precipitation in step 4, resuspend (osmotic pressure 260 ± 20mOsm / kgH 2 O), after fixing at room temperature for 5 hours, wash with PBS buffer to remove the fixative.
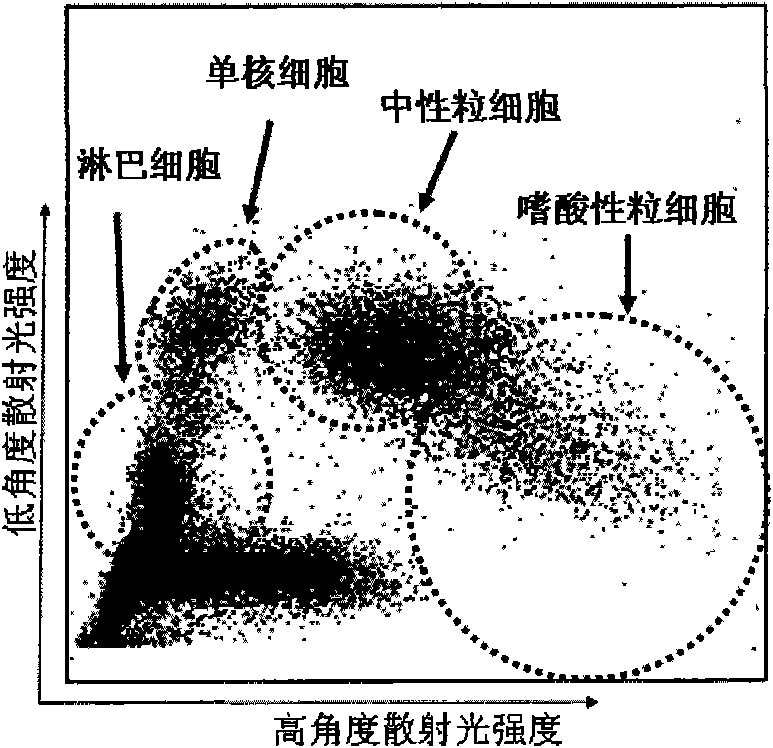
[0060] The eosinophil simulant prepared in step 5 was detected by a blood cell analyzer that simultaneously detects low-angle scattered light and high-angle scattered light, and the results were as follows image 3 shown.
Embodiment 2
[0062] 1. Take 100ml of fresh anticoagulated bovine blood, containing 2g / L of anticoagulant;
[0063] 2. Mix 100ml of anticoagulated bovine blood with 400ml of hemolytic agent, stir for 10 minutes, centrifuge at 2000g for 5 minutes, and harvest white blood cells;
[0064] 3. Centrifuge and wash white blood cells with PBS buffer once or twice;
[0065] 4. Take the white blood cells in step 3 and use PBS to adjust the white blood cell count to 50×10 9 pieces / L, centrifugal force 200g, centrifuge for 10 minutes, harvest the precipitate;
[0066] 5. Get the precipitation in step 4, resuspend (osmotic pressure 1400 ± 20mOsm / kgH 2 O), after fixing at room temperature for 5 hours, wash with PBS buffer to remove the fixative.
[0067] The eosinophil simulant prepared in step 5 was detected by a blood cell analyzer that simultaneously detects low-angle scattered light and high-angle scattered light, and the results were as follows Figure 4 shown.
Embodiment 3
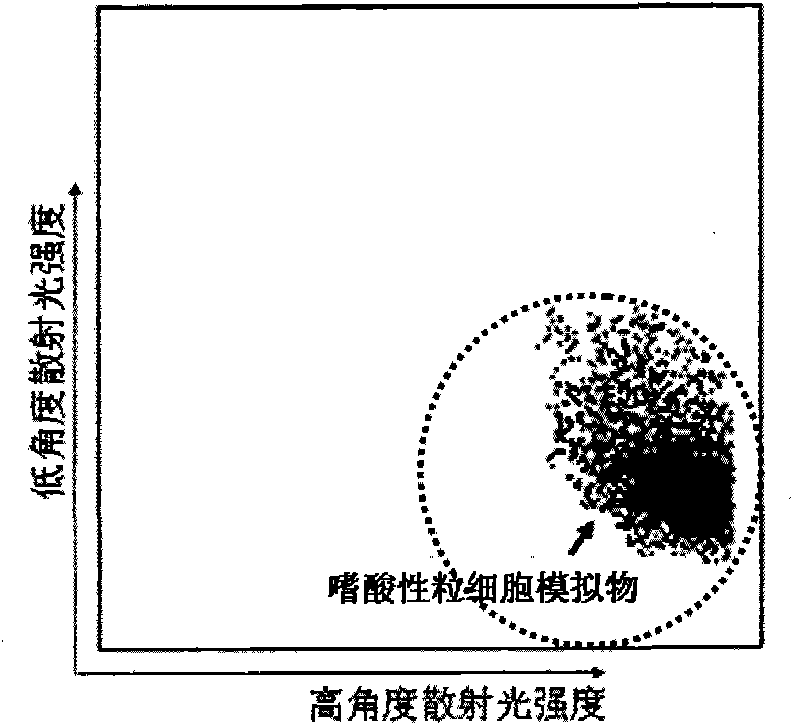
[0069] 1. Take 100ml of fresh anticoagulated bovine blood, containing 2g / L of anticoagulant;
[0070] 2. Mix 100ml of anticoagulated bovine blood with 400ml of hemolytic agent, stir for 10 minutes, centrifuge at 2000g for 5 minutes, and harvest white blood cells;
[0071] 3. Centrifuge and wash white blood cells with PBS buffer once or twice;
[0072] 4. Take the white blood cells in step 3 and use PBS to adjust the white blood cell count to 50×10 9 pieces / L, centrifugal force 200g, centrifuge for 10 minutes, harvest the precipitate;
[0073] 5. Get the precipitation in step 4, resuspend (osmotic pressure 400 ± 20mOsm / kgH 2 O), after fixing at room temperature for 5 hours, wash with PBS buffer to remove the fixative.
[0074] The eosinophil simulant prepared in step 5 was detected by a blood cell analyzer that simultaneously detects low-angle scattered light and high-angle scattered light, and the results were as follows Figure 5 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com