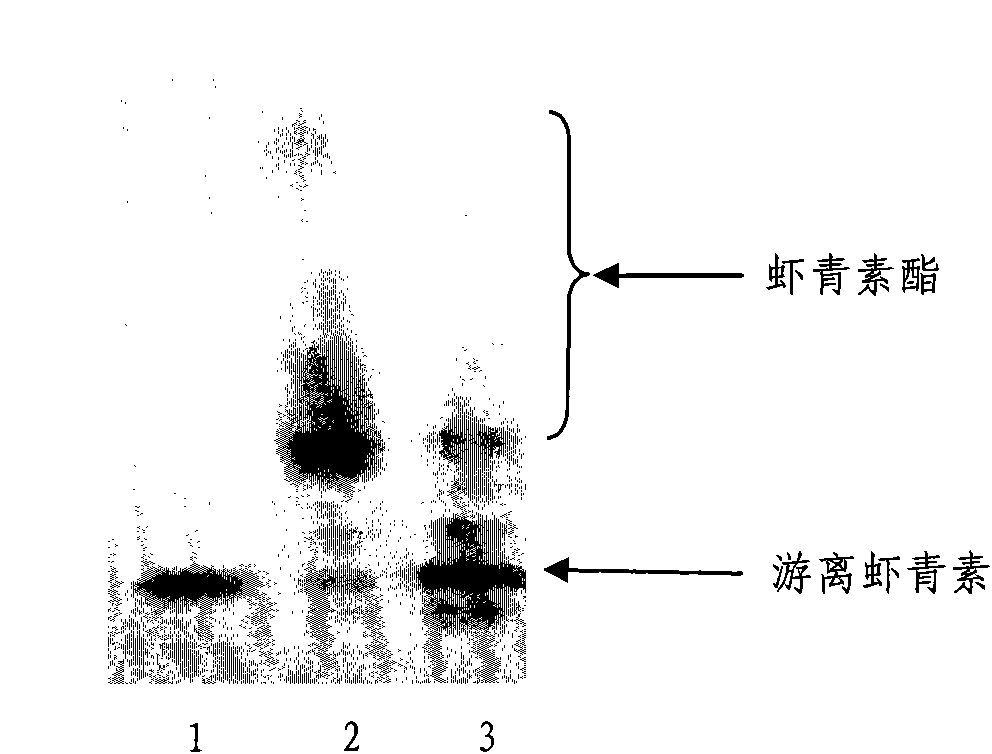
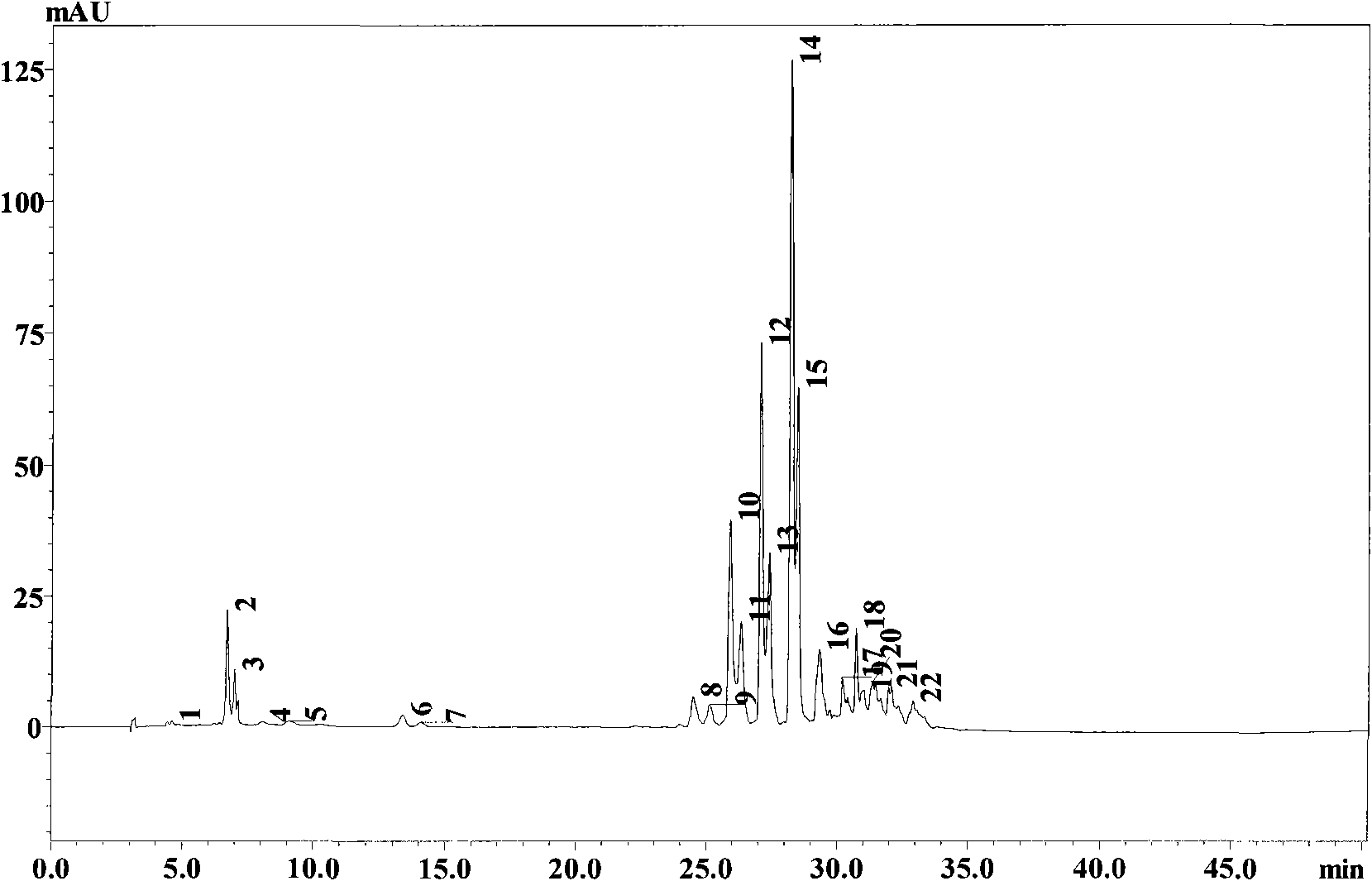
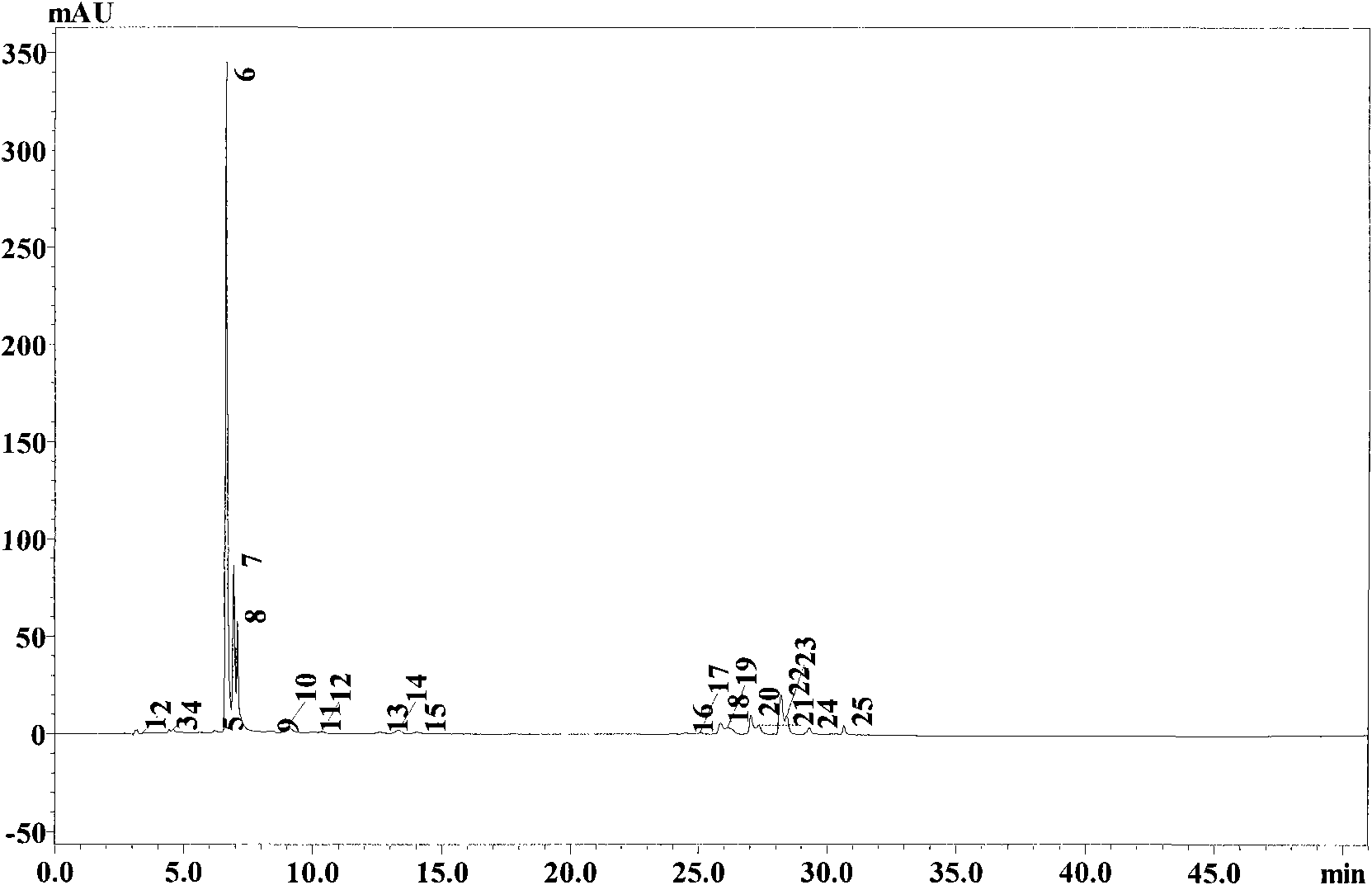
Method for preparing astaxanthin monomer
A technology of astaxanthin and astaxanthin ester, applied in the biological field, can solve the problems of low yield, difficult conditions, etc., and achieve the effects of simple operation, energy saving, and avoiding the loss of astaxanthin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The preparation of embodiment 1Penicillium cyclopium var.albus alkaline lipase enzyme liquid
[0021] With the cDNA of Penicillium cyclopium var.albus (CGMCC 3.4041) alkaline lipase as a template, primers (P1: gaattc gcaactgctgacgccgct, P2: gcggccg tcagctcagatagccaca) for PCR amplification. The target band was recovered from the PCR product, and connected to the vector pMD18-T-Simple (purchased from TaKaRa Company); then double-digested pMD18-T-alip and pKIC9K with restriction endonuclease SnaBI and Not 1 respectively , and then the target fragment was recovered and ligated with T4 ligase to obtain the expression plasmid pPIC9K-alip. Digest the carrier PIC9K-alip with restriction endonuclease Sal I, then concentrate the linearized fragment by ethanol precipitation, transform Pichia pastoris GS115 competent cells by electroshock method, and spread the histidine-free cells after transformation Minimal Medium MD Plates. After the plates were cultured at 30°C for 2-3 ...
Embodiment 2
[0023] Example 2 Emulsion Preparation and Enzymolysis Reaction Condition Optimization
[0024] Take 0.1g Haematococcus pluvialis astaxanthin crude extract oil (natural L-astaxanthin 5% oil, purchased from Jingzhou Natural Astaxanthin Co., Ltd.) Warm at 80°C, grind and emulsify, and add 0.1M sodium phosphate buffer (pH5.0-8.0) to make up to 10ml; add 2-12U lipase per microgram of total carotenoids and add emulsified product (1ml) and enzyme, Then add 0.1M sodium phosphate buffer solution with pH 5.0-8.0 to a total volume of 10ml; shake the reaction at 30°C at 180rpm, take samples every 1-2h, detect with TLC and HPLC, and stop the reaction after 12h.
[0025] The results show that Tween 80 can be effectively emulsified by adding 1 to 3 times the weight of the oil agent of the crude extract of Haematococcus pluvialis, but because the amount of Tween 80 will increase the amount of acetone in the extract, considering economic factors, the amount of Tween 80 is preferred It is 1 ti...
Embodiment 3
[0026] The hydrolysis of embodiment 3 astaxanthin esters
[0027] Take 0.1g of Haematococcus pluvialis astaxanthin crude extract oil (purchased from Jingzhou Natural Astaxanthin Co., Ltd.) in a mortar, add 1 times the mass of Tween 80, grind and emulsify, and add 0.1M sodium phosphate Buffer (pH7.0) to 10ml; add emulsification (1ml) and enzyme at the amount of 4U lipase per microgram of total carotenoids, then add 0.1M sodium phosphate buffer at pH7.0 to the total volume 10ml; 28°C, 150rpm shaking reaction for 7 hours, the yield of astaxanthin was 63.2%. After the reaction, add the same volume of acetone as the sample, shake with a vortex shaker for 30s at room temperature, then add an equal volume of n-hexane, shake for 30s in the same way as above, 12000rpm, centrifuge for 1min, and take the supernatant for TLC and HPLC detection .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com