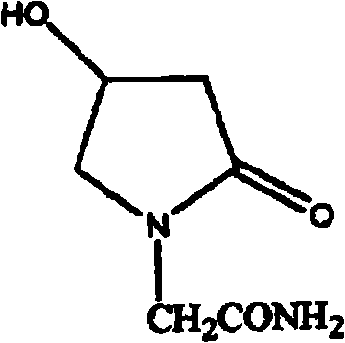
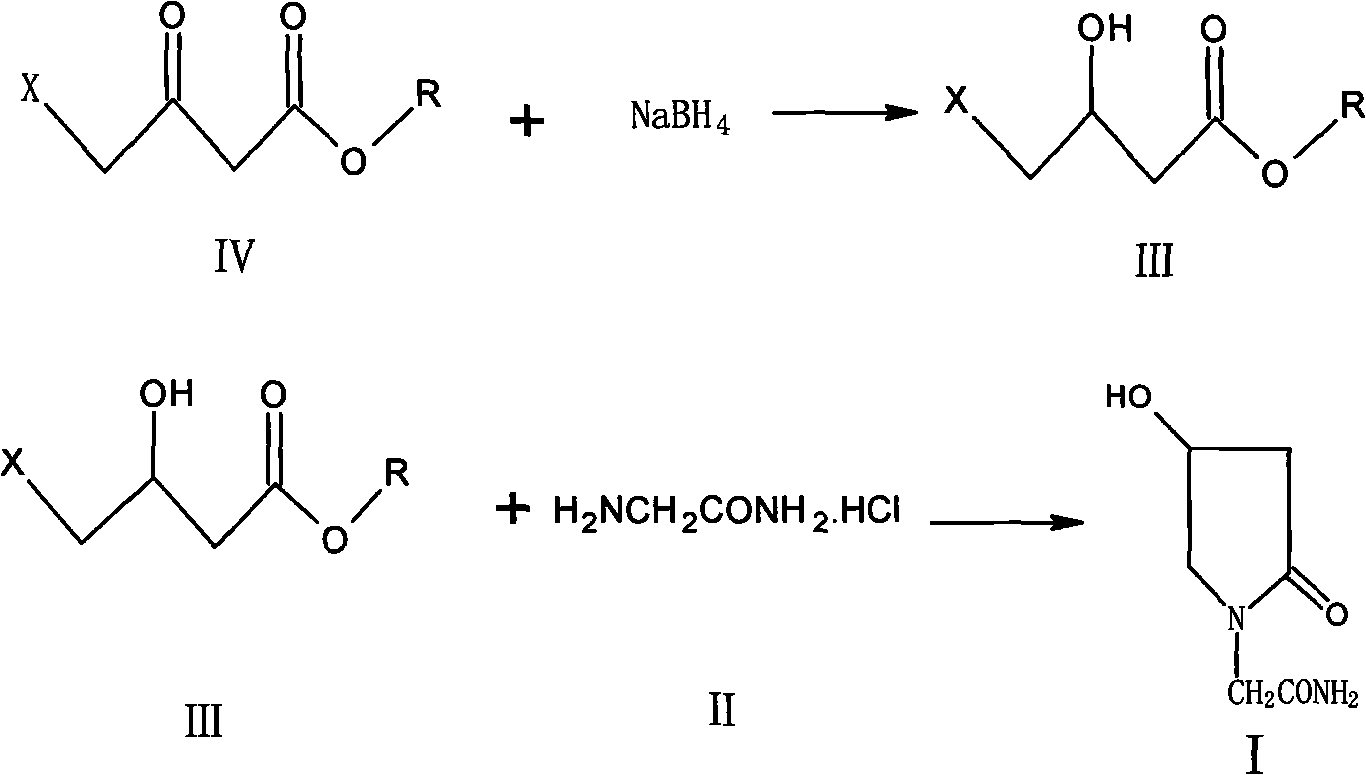
Preparation method of 4-hydroxyl ketopyrrolidine-2-acetamide
A technology of hydroxypyrrolidone and acetamide, which is applied in the field of synthesis of 4-hydroxypyrrolidone-2-acetamide, can solve the problems of low total yield, many reaction steps, low atom economy and the like, and achieves reduction of treatment process and total yield. The effect of improving efficiency, shortening process route and reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0026] The present invention will be further described below in conjunction with the examples, but not limited to the examples.
[0027] Example step 1
[0028] Preparation of methyl 4-chloro-3-hydroxybutyrate
[0029] In a 2L four-necked bottle, install a dropping funnel, a thermometer, stir, add KBH418.5g, add industrial ethanol 1700ml, stir well to make it mix and suspend. Add 163g of methyl 4-chloroacetoacetate dropwise to the above solution. After the dropwise addition, stir at 32-35°C for 6 hours and filter. The filtrate was concentrated under reduced pressure to remove the solution, the obtained oil was extracted with 150ml of saturated brine, the oil layer was separated, the water layer was extracted twice with 80ml of dichloromethane, the organic layers were combined, dried with 15g of anhydrous sodium sulfate, concentrated to recover dichloromethane , 140.8 g of methyl 4-chloro-3-hydroxybutyrate was obtained.
[0030] Example step 2
[0031] Preparation of Oxirac...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com