Preparation method of genipin
A technology of genipin and preparation process, applied in microorganism-based methods, biochemical equipment and methods, immobilized on/in organic carriers, etc., can solve the problem that genipin is expensive and reduces the yield of genipin , the environment is easy to cause pollution and other problems, to achieve the effect of low cost, improve yield and overcome high cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Ferment the enzyme-producing Aspergillus niger strain for 96 hours, take out the fermented liquid together with mycelium, and concentrate it with a hollow fiber ultrafilter with a molecular weight of 6000 to about one-third of the original volume, and add the final The gelatin with a concentration of 1% and the glutaraldehyde with a final concentration of 0.25% were cross-linked, the cross-linking time was 1 hour, and the cross-linking temperature was 10°C;
[0037] (2) Embedding the cross-linked enzyme and mycelia liquid with sodium alginate, the concentration of sodium alginate being 3%;
[0038] (3) Add the embedded mixed solution dropwise to the calcium chloride solution for fixation, the concentration of calcium chloride is 3%, and prepare immobilized enzyme pellets;
[0039] (4) Place the prepared immobilized enzyme pellets at room temperature in 0.5% calcium chloride solution for hardening, and the hardening time is 2 hours;
[0040] (5) Filter the hardened ...
Embodiment 2
[0044] (1) Ferment the enzyme-producing Aspergillus Usami strain for 120 hours, take out the fermented liquid together with mycelia, concentrate it with a hollow fiber ultrafilter with a molecular weight of 30,000, and concentrate it to about one-fifth of the original volume, and add the final Concentration is 0.5% chitosan and final concentration is 0.5% glutaraldehyde to carry out cross-linking, cross-linking time is 2 hours, and cross-linking temperature is 20 ℃;
[0045] (2) Embedding the cross-linked enzyme and mycelia liquid with sodium alginate, the concentration of sodium alginate being 3%;
[0046] (3) Add the embedded mixed solution dropwise to the calcium chloride solution for fixation, the concentration of calcium chloride is 3%, and prepare immobilized enzyme pellets;
[0047] (4) Place the prepared immobilized enzyme beads at room temperature in 0.5% calcium chloride solution for hardening, and the hardening time is 3 hours;
[0048] (5) Filter the hardened immo...
Embodiment 3
[0052] (1) Ferment the enzyme-producing Rhizopus oryzae strain for 96 hours, take out the fermented liquid together with mycelia, add a final concentration of 2% gelatin and a final concentration of 1% glutaraldehyde for cross-linking, and the cross-linking time is 1 hour , the crosslinking temperature is 10°C;
[0053] (2) Embedding the cross-linked enzyme and mycelia liquid with sodium alginate, the concentration of sodium alginate being 3%;
[0054] (3) Add the embedded mixed solution dropwise to the calcium chloride solution for fixation, the concentration of calcium chloride is 4%, and prepare immobilized enzyme pellets;
[0055] (4) Place the prepared immobilized enzyme pellets at room temperature in 0.5% calcium chloride solution for hardening, and the hardening time is 2 hours;
[0056] (5) Filter the hardened immobilized enzyme and store it at 4°C for later use;
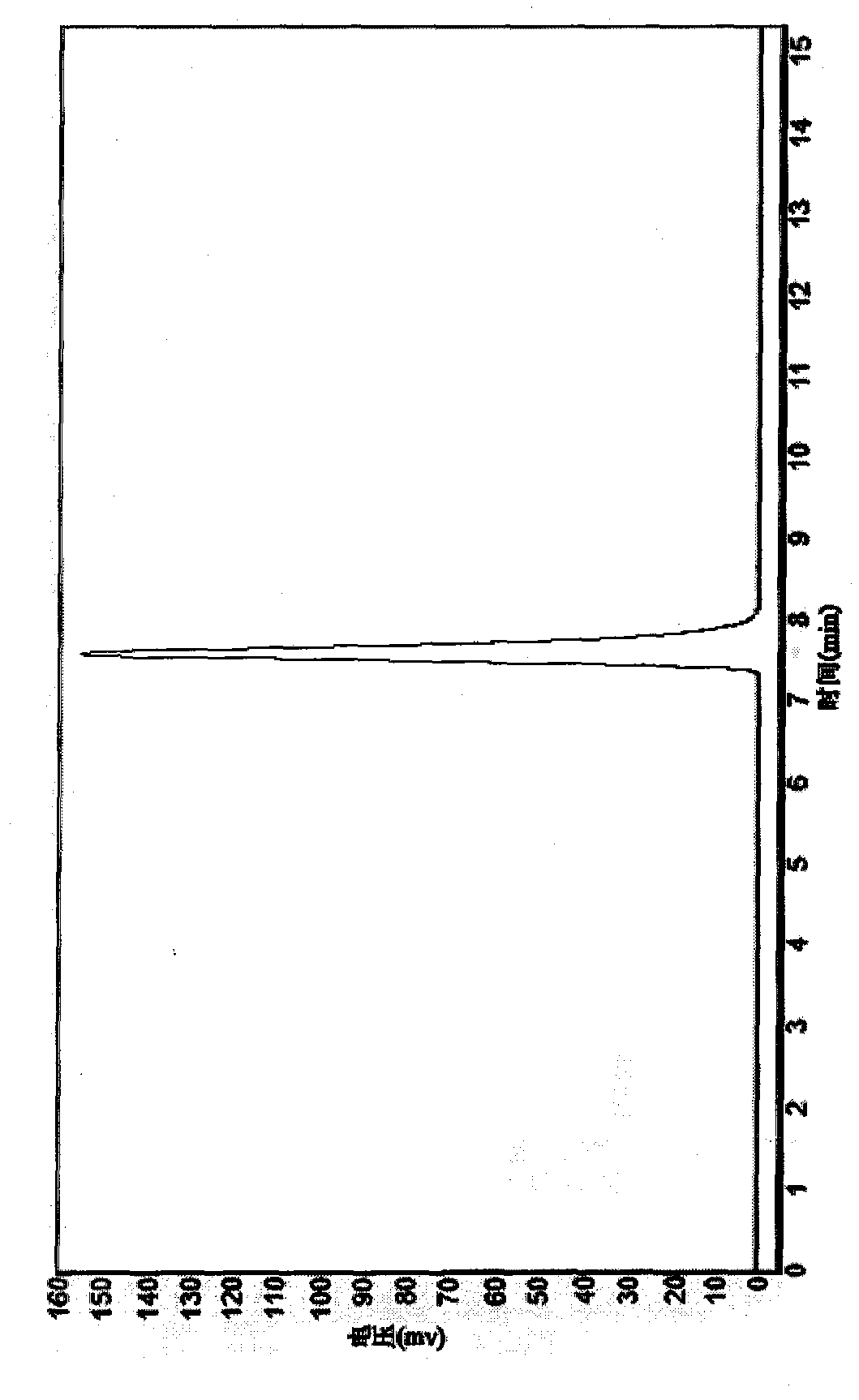
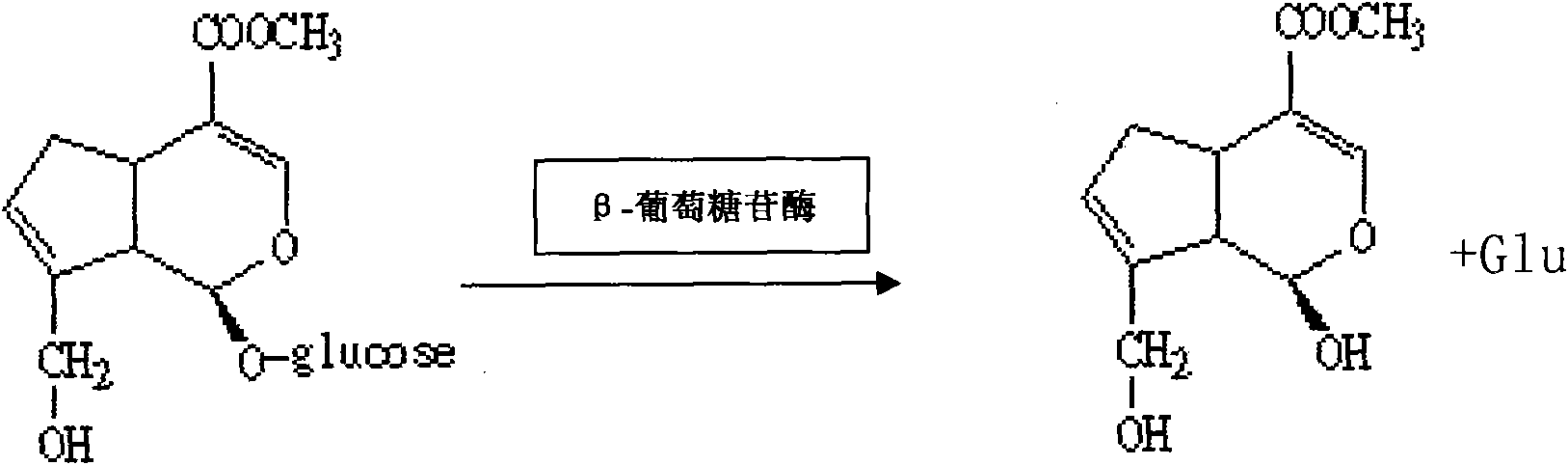
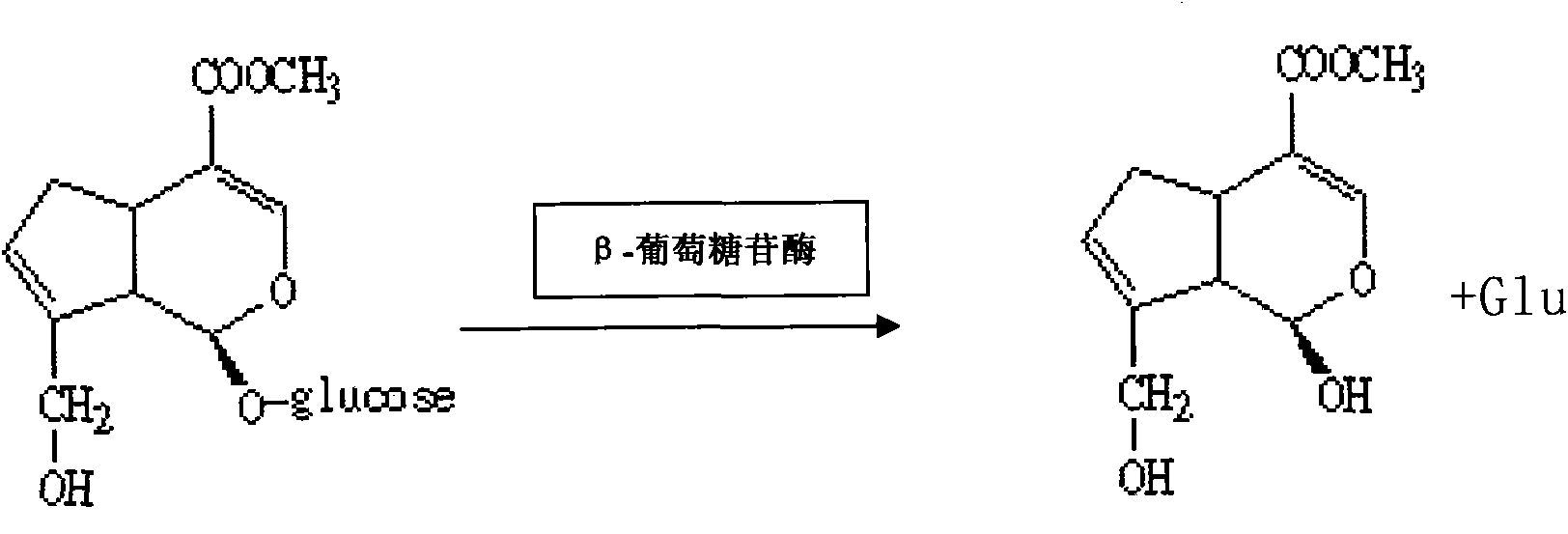
[0057] (6) Wet-pack the immobilized enzyme into a column, then prepare 2% geniposide solution, adjust t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com